��Ŀ����
[���ʽṹ������]��֪��A��B��C��D��E��F����Ԫ�أ�ԭ��������������Aԭ�Ӻ�����������״�ĵ����ƣ�������״�ĵ����ƹ���ϵ�������ȣ�B�Ƕ�������ԭ�Ӱ뾶����Ԫ�أ�CԪ��3p�ܼ��������E���������ڵ縺������Ԫ�أ�F�ǵ�������δ�ɶԵ�������Ԫ�ء�
�Իش������йص����⣺
��1��д��FԪ�صĵ����Ų�ʽ��________________��
��2����֪AԪ�ص�һ���⻯������к��ĸ�ԭ�ӣ����ڸû�����ķ�����Aԭ�ӵ��ӻ��������Ϊ__________��
��3����֪C��E����Ԫ���γɵĻ�����ͨ����CE3��CE5���֡������ֻ�������һ��Ϊ�Ǽ��Է��ӣ�һ��Ϊ���Է��ӣ����ڼ��Է��ӵĻ�����ķ������幹����________________��
��4��B��C��D��E�ĵ�һ�������ɴ�С��˳����________��дԪ�ط��ţ�������Ԫ������������ˮ�����γɵ���Һ�����ʵ���Ũ����ͬʱ��pH�ɴ�С��˳����________________��д��ѧʽ����
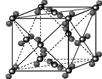
��5����B��E��Ԫ���γɵĻ�������ɵľ����У����������Ӷ��������ͶԳƽṹ�����Ƕ����Կ�������Բ���˴ˡ����С�������ͼ��ʾΪB��E�γɻ�����ľ����ṹͼ�Լ�����������ͼ��
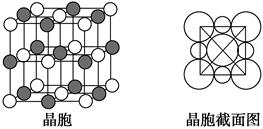
�����о���һ��B�������B����________�����������ܶ�Ϊ�� g��cm��3�������ӵ�������ֵ��NA��ʾ����E�������Ӱ뾶Ϊ________cm���ú�NA��ѵ�ʽ�ӱ����
�Իش������йص����⣺
��1��д��FԪ�صĵ����Ų�ʽ��________________��
��2����֪AԪ�ص�һ���⻯������к��ĸ�ԭ�ӣ����ڸû�����ķ�����Aԭ�ӵ��ӻ��������Ϊ__________��
��3����֪C��E����Ԫ���γɵĻ�����ͨ����CE3��CE5���֡������ֻ�������һ��Ϊ�Ǽ��Է��ӣ�һ��Ϊ���Է��ӣ����ڼ��Է��ӵĻ�����ķ������幹����________________��
��4��B��C��D��E�ĵ�һ�������ɴ�С��˳����________��дԪ�ط��ţ�������Ԫ������������ˮ�����γɵ���Һ�����ʵ���Ũ����ͬʱ��pH�ɴ�С��˳����________________��д��ѧʽ����
��5����B��E��Ԫ���γɵĻ�������ɵľ����У����������Ӷ��������ͶԳƽṹ�����Ƕ����Կ�������Բ���˴ˡ����С�������ͼ��ʾΪB��E�γɻ�����ľ����ṹͼ�Լ�����������ͼ��

�����о���һ��B�������B����________�����������ܶ�Ϊ�� g��cm��3�������ӵ�������ֵ��NA��ʾ����E�������Ӱ뾶Ϊ________cm���ú�NA��ѵ�ʽ�ӱ����
��1��1s22s22p63s23p63d54s1��[Ar]3d54s1
��2��sp3����3��������
��4��Cl>P>S>Na��NaOH>H3PO4>HClO4>H2SO4
��5��12��
��2��sp3����3��������
��4��Cl>P>S>Na��NaOH>H3PO4>HClO4>H2SO4
��5��12��

A��B��C��D��E��F����Ԫ�أ�ԭ���������������ɡ�Aԭ�Ӻ�����������״�ĵ����ƣ�������״�ĵ����ƹ���ϵ�������ȡ��Ƴ�AΪO���ɡ�B�Ƕ�������ԭ�Ӱ뾶����Ԫ�ء��Ƴ�BΪNa���ɡ�CԪ��3p�ܼ���������Ƴ�CΪP���ɡ�F�ǵ�������δ�ɶԵ�������Ԫ�ء��Ƴ�FΪCr���ɡ�E���������ڵ縺������Ԫ�ء���ԭ��������С��ϵ�Ƴ�EΪCl����DΪS��
��2�����ĸ�ԭ�ӵ�A���⻯��ΪH2O2������ԭ�ӵ��ӻ��������Ϊsp3��
��3��CE3��CE5ΪPCl3��PCl5��PCl3�Ǽ��Է��ӣ��ռ乹��Ϊ�����Σ�PCl5Ϊ�Ǽ��Է��ӡ�
��4��ͬ��������Ԫ�ص�һ�����ܴ���������������ƣ����ڢ�A�巴������һ�������ɴ�С��˳����Cl>P>S>Na������Ԫ������������Ӧ��ˮ����ֱ���NaOH��H3PO4��H2SO4��HClO4��NaOH�ǼH3PO4����ǿ�ᣬH2SO4��HClO4����ǿ�ᣬ���ʵ���Ũ����ͬʱ��pH�ɴ�С˳��ΪNaOH>H3PO4>HClO4>H2SO4��
��5����Na�������Na������12�����������Σ���������ͼ���ı߳�Ϊa cm��Cl���İ뾶Ϊr cm����a3�� ����a��
����a�� ��r��
��r�� ��
��
��2�����ĸ�ԭ�ӵ�A���⻯��ΪH2O2������ԭ�ӵ��ӻ��������Ϊsp3��
��3��CE3��CE5ΪPCl3��PCl5��PCl3�Ǽ��Է��ӣ��ռ乹��Ϊ�����Σ�PCl5Ϊ�Ǽ��Է��ӡ�
��4��ͬ��������Ԫ�ص�һ�����ܴ���������������ƣ����ڢ�A�巴������һ�������ɴ�С��˳����Cl>P>S>Na������Ԫ������������Ӧ��ˮ����ֱ���NaOH��H3PO4��H2SO4��HClO4��NaOH�ǼH3PO4����ǿ�ᣬH2SO4��HClO4����ǿ�ᣬ���ʵ���Ũ����ͬʱ��pH�ɴ�С˳��ΪNaOH>H3PO4>HClO4>H2SO4��
��5����Na�������Na������12�����������Σ���������ͼ���ı߳�Ϊa cm��Cl���İ뾶Ϊr cm����a3��
 ����a��
����a�� ��r��
��r�� ��
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ



 ��X��Y�γɵ�һ�ֻ����ﻥΪ�ȵ�����,��
��X��Y�γɵ�һ�ֻ����ﻥΪ�ȵ�����,��

 �����幹��Ϊ����������
�����幹��Ϊ����������