��Ŀ����
ˮ������֮Դ���������ǵ�����������ء��ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ���
��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2��д����H2O���ӻ�Ϊ�ȵ�������� ����2�֣���
��3��ˮ�������ض����������õ�һ��H�����γ�ˮ�������ӣ�H3O���������ж��������̵��������������� ��
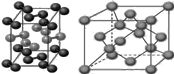
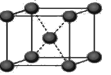
��4���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ(δ��˳������)������ľ���������ͬ����
(������Ӧ�ı����ĸ��д)��
A B C D E
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ��������֪������������51 kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»���(11 kJ/mol)���������������ġ����ܡ��� kJ/mol��
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӡ���д�����ɴ�������ӵ����ӷ���ʽ�� ��
��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2��д����H2O���ӻ�Ϊ�ȵ�������� ����2�֣���
��3��ˮ�������ض����������õ�һ��H�����γ�ˮ�������ӣ�H3O���������ж��������̵��������������� ��
| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |
(������Ӧ�ı����ĸ��д)��




A B C D E
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ��������֪������������51 kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»���(11 kJ/mol)���������������ġ����ܡ��� kJ/mol��
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӡ���д�����ɴ�������ӵ����ӷ���ʽ�� ��
��1��1S22S22P4 (1��) ��2��H2S��NH2��(2��) ��3��A (1��) ��4��BC (2��)
��5��20 (1��) ��6��Cu2++4H2O��[Cu(H2O)4]2+(1��)
��5��20 (1��) ��6��Cu2++4H2O��[Cu(H2O)4]2+(1��)
�����������1����ԭ�ӵĺ˵������8�����ݹ���ԭ����֪��Χ������6�����ӣ�2s�ܼ�����2�����ӣ�2p�ܼ�����4�����ӣ�����ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ1S22S22P4��
��2��ԭ�Ӹ�����ȡ��۵�������ȵ���Ϊ�ȵ����塣ˮ���Ӻ���3��ԭ�ӡ�8���۵��ӣ������ˮ��Ϊ�ȵ���������У�H2S��NH2-��
��3��A��ˮ��������ԭ�Ӻ���2�����۵�����2���µ��Ӷԣ���ռ乹����V�ͣ�����ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3������ԭ�ӵ��ӻ�����û�иı䣬��A����ȷB��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ���������״�����˸ı䣬��B������C����ṹ��ͬ�������ʲ�ͬ�����Ļ�ѧ���ʷ����˸ı䣬��C������D��ˮ����ΪV�ͣ�������104.5�㡣H3O+Ϊ�����ͣ����еļ��Ƿ����˸ı䣬��D��������˴�Ϊ��A��
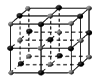
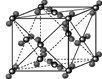
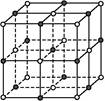
��4�������ڷ��Ӿ��壬�������ľ����ṹ�ص��֪��A�����Ӿ����Ȼ��Ƶľ����������������ӡ�BΪ�ɱ��ľ���ͼ��������Ϊ���ӣ����ڷ��Ӿ��塣CΪ��ľ���ͼ��������Ϊ����ӣ����Է��Ӿ��壻D��ԭ�Ӿ�����ʯ�ľ�������������ԭ�ӡ�E�ǽ��������Ƶľ������������ǽ��������Ӻ����ɵ��ӣ�������ľ���������ͬ���Ǹɱ��͵ⵥ�ʣ��ʴ�Ϊ��BC��
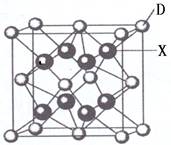
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ����������ݽṹͼ
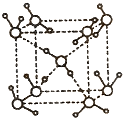 ��֪��1molˮ�к���2mol����������ȣ����»���+��������ڱ�����������51kJ/mol��ˮ���Ӽ�ķ��»�����11kJ/mol�����Ա�����������ġ����ܡ���(51kJ/mol��11kJ/mol)��2��20kJ/mol��
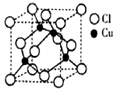
��֪��1molˮ�к���2mol����������ȣ����»���+��������ڱ�����������51kJ/mol��ˮ���Ӽ�ķ��»�����11kJ/mol�����Ա�����������ġ����ܡ���(51kJ/mol��11kJ/mol)��2��20kJ/mol����6����ˮ����ͭ����ˮ������ˮ��ͭ���ӣ�ͭ�����ṩ�չ����ˮ�����е���ԭ���ṩ�µ��Ӷԣ��γ���λ������������ӵĽṹ��
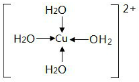 �����ɴ�������ӵ����ӷ���ʽCu2++4H2O��[Cu(H2O)4]2+��
�����ɴ�������ӵ����ӷ���ʽCu2++4H2O��[Cu(H2O)4]2+��
��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ


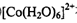 �ڹ�����ˮ����ת��Ϊ
�ڹ�����ˮ����ת��Ϊ ��д��
��д�� �ļ۲�����Ų�ͼ____��
�ļ۲�����Ų�ͼ____��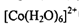 ��
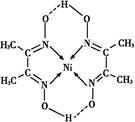
��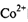 ����λ��Ϊ____��NH3���ӵ�����ԭ���ӻ���ʽΪ____��
����λ��Ϊ____��NH3���ӵ�����ԭ���ӻ���ʽΪ____��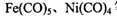 �ȡ�CO��N2���ڵȵ����壬��CO������
�ȡ�CO��N2���ڵȵ����壬��CO������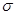 ����
����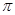 ����Ŀ��Ϊ____��д����CO��Ϊ�ȵ������һ�������ӵ����ӷ���____��
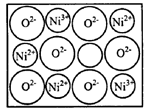
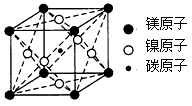
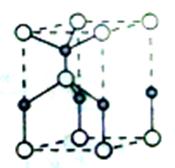
����Ŀ��Ϊ____��д����CO��Ϊ�ȵ������һ�������ӵ����ӷ���____��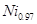 O���þ�����Ni3����Ni2����������֮��Ϊ____��
O���þ�����Ni3����Ni2����������֮��Ϊ____��