��Ŀ����
18�����м��־������ĺ��������һ�������¿����ת�����밴Ҫ������
��1�����������У���Ϊͬ���칹����Ǣڢݢߣ�����ţ�����Ϊͬϵ����Ǣۢܣ�����ţ���
��2��һ�������¢ٿ�ת��Ϊ�ڣ��÷�Ӧ���ڣ��������Ӧ���ͣ��������Լ�����ʵ�ָ�ת������C��
A�����������Һ�� �� B����ˮ������ C��������Һ���� ��D������������Һ
��3����д���������������Ģٵ�ͬ���칹��
 ��
��a�������г������ⲻ��������״�ṹ��b��������ˮ1��5��Ӧ��c��������һ�ȴ������֣�
��4����д����������NaOH��Һ��һ�������·�Ӧ�Ļ�ѧ����ʽ
 +2nNaOH��n
+2nNaOH��n +��n+1��H2O��
+��n+1��H2O��
���� ��1��������ͬ����ʽ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻�ṹ���ơ��������������ɸ���CH2��ԭ���ŵ��л������ﻥ���Ϊͬϵ��ݴ˻ش�
��2��һ�������¢�ת��Ϊ�ڵķ�Ӧʽȩ��ת��Ϊ�Ȼ��ķ�Ӧ����ҪѰ������������ʵ�֣�
��3������ͬ���칹��ĸ����Լ���д�������ش�
��4�����������������Ƶ������»ᷢ��ˮ�ⷴӦ���õ��������κͷ�������Һ���ݴ���д��
��� �⣺��1���ڢݢ߾�����ͬ����ʽ���ṹ��ͬ����Ϊͬ���칹�壻�ۢܽṹ���ơ��������������ɸ���CH2��ԭ���ţ�Ϊͬϵ��ʴ�Ϊ���ڢݢߣ��ۢܣ�
��2��һ�������¢�ת��Ϊ�ڵķ�Ӧʽȩ��ת��Ϊ�Ȼ��ķ�Ӧ������������Ӧ����ҪѰ������������ʵ�֣����������Բ���̫ǿ������ѡ��������Һ����ˮ�Լ�������ػὫ���е�̼̼˫����Ӧ�����������Ʋ����������ԣ��ʴ�Ϊ��������Ӧ��C��
��3����������a�������г������ⲻ��������״�ṹ��b��������ˮ1��5��Ӧ��c��������һ�ȴ������ֵĢٵ�ͬ���칹���ǣ� ��
��
�ʴ�Ϊ�� ��
��
��4�� ������NaOH��Һ��һ�������·�Ӧ�Ļ�ѧ����ʽΪ
������NaOH��Һ��һ�������·�Ӧ�Ļ�ѧ����ʽΪ +2nNaOH��n
+2nNaOH��n +��n+1��H2O���ʴ�Ϊ��
+��n+1��H2O���ʴ�Ϊ�� +2nNaOH��n
+2nNaOH��n +��n+1��H2O��
+��n+1��H2O��
���� �����ۺϿ���ѧ��ͬ���칹��ĸ������д�Լ����ʵ�����֪ʶ��ע��֪ʶ�Ĺ��ɺ������ǽ���Ĺؼ����Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���NaHCO3����Al2O3��NH4HSO3������ ��Al��
| A�� | �ۢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �٢ڢۢ� |
| A�� | ����B������������Ũ���� | |
| B�� | A���⻯��е����C���⻯���ΪA�ķǽ����Ա�Cǿ | |
| C�� | C��D�γɵĻ�����CD2�����и�ԭ�Ӷ�����8�����ȶ��ṹ | |
| D�� | Ԫ��A��C�γɵĻ�����CA2��ˮ��Һ��ͨ��D���ʳ�ַ�Ӧ����Һ���Լ��� |
| A�� | ������Һ����մ��Ƥ���ϣ������þƾ���Һϴ�� | |
| B�� | �κ������£����ݲ�����ת��Ϊ�����ǻ�ƾ� | |
| C�� | �Ȼ�����HgCl2����ϡ��Һ��������������е��������Ϊ����ʹ���嵰���ʱ��� | |
| D�� | ŨHNO3����Ƥ���ϣ�ʹƤ���ʻ�ɫ��������ŨHNO3�͵����ʷ�����ɫ��Ӧ |
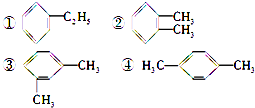
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
| A�� | c��H+����c��OH-�� | B�� | c��CH3COOH��+c��CH3COO-��=0.2mol/L | ||
| C�� | c��CH3COOH����c��CH3COO-�� | D�� | c��CH3COO-��+c��OH-��=0.1mol/L |