��Ŀ����
9����ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100g 5.00%��NaOH��Һ��������CuSO4��Һ��100g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��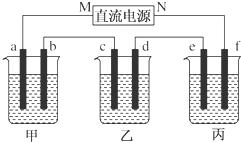
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������d�缫�������ӣ��ݴ˻ش����⣺
��1����Դ��N��Ϊ������
��2���缫a�Ϸ����ĵ缫��ӦΪ2H++2e-=H2����
��3����ʽ����缫a�����ɵ������ڱ�״���µ������5.6L
��4���缫c�������仯��16g��
��5�����ǰ�����Һ���ᡢ���Դ�С�仯���
����Һ��������
����Һ��������
����Һ����Դ�Сû�б仯��
���� ��1��������C�缫�������ӣ���c�������ķ�ӦΪ��Cu2++2e-=Cu����C��Ϊ�������ɴ˿��Ƴ�bΪ������aΪ������MΪ������NΪ����������ΪK2SO4���൱�ڵ��ˮ�������ˮ������Ϊx���ɵ��ǰ��������������У�100��10%=��100-x����10.47%����x=4.5g����Ϊ0.25mol���ɷ���ʽ2H2+O2�T2H2O��֪������2molH2O��ת��4mol���ӣ�����������Ӧ��ת��0.5mol���ӣ���������·�Ǵ����ģ���ÿ���ձ��еĵ缫��ת�Ƶ���������ȵģ�
��2������ΪNaOH���൱�ڵ��H2O������a��Ϊ�����ӷŵ磻
��3��ת��0.5mol���ӣ����Ը��ݵ缫��Ӧʽ������������������
��4��Cu2++2e-=Cu��ת��0.5mol���ӣ��ݵ缫��Ӧʽ���ش�
��5�������൱�ڵ��ˮ����NaOH��Ũ������pH�����������ΪCu2+�ŵ磬����ΪOH-�ŵ磬����H+���࣬��pH��С������Ϊ���ˮ������K2SO4���ԣ���pH�������䣮
��� �⣺��1�����ұ���c�������ӣ�˵��Cu������c�缫�ϣ������Ǵ�b-c�ƶ���M�Ǹ�����NΪ�������ʴ�Ϊ������
�ڼ���ΪNaOH���൱�ڵ��H2O������a��Ϊ������H+�ŵ磬��2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�۱���ΪK2SO4���൱�ڵ��ˮ�������ˮ������Ϊx���ɵ��ǰ��������������У�100��10%=��100-x����10.47%����x=4.5g����Ϊ0.25mol���ɷ���ʽ2H2+O2�T2H2O��֪������2molH2O��ת��4mol���ӣ�����������Ӧ��ת��0.5mol���ӣ�������H2Ϊ$\frac{0.5}{2}$mol=0.25mol������µ����Ϊ0.25��22.4L=5.6L��
�ʴ�Ϊ��5.6��
��4��������·�Ǵ����ģ�����ÿ���ձ��еĵ缫��ת�Ƶ���������ȵģ�����c���������缫��Ӧ��Cu2++2e-=Cu����֪ת��0.5mol�������ɵ�m��Cu��=$\frac{0.5mol}{2}$��64=16g��
�ʴ�Ϊ��16��
��5�������൱�ڵ��ˮ����NaOH��Ũ������pH�����������ΪCu2+�ŵ磬����ΪOH-�ŵ磬��ⷽ��ʽΪ��2CuSO4+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+2H2SO4������H+���࣬��pH��С������Ϊ���ˮ������K2SO4���ԣ���pH�������䣬
�ʴ�Ϊ����������������������Դ�Сû�б仯��
���� ����Ϊ�绯ѧ֪ʶ���ۺ�Ӧ�ã�����ʱҪע����ݵ缫��Ӧ�����жϳ����ص��������������жϳ���Դ����������Ҫע����������Ϊ������·�����缫�ϵ�ʧ���ӵ���Ŀ��ȣ�����ʱҪ��ȷд���缫����ʽ��ȷ�ж����������ӵķŵ�˳��

 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�| A�� | ��ϩ�ͻ����� | B�� | ��ϩ�ͼ�ϩ | C�� | ����ͱ� | D�� | ��������춡�� |

����˵����ȷ���ǣ�������
| A�� | ����ͪ��ѧʽΪC9H12O | |
| B�� | Cyrneine A���Է����ӳɷ�Ӧ����ȥ��Ӧ��������Ӧ | |
| C�� | ����ͪ��Cyrneine A����ʹ����KMnO4��Һ��ɫ | |
| D�� | ������ͪ��Ϊͬ���칹�壬��������4�ֲ�ͬ��ѧ��������ԭ�ӵķ�����ﹲ��4�� |
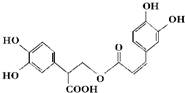
| A�� | �Ե��������ڷ����� | |
| B�� | 1 mol�Ե���������ܺ�9 mol���������ӳɷ�Ӧ | |
| C�� | �Ե�������Է���ˮ�ⷴӦ��ȡ����Ӧ��������Ӧ | |
| D�� | 1 mol�Ե���������ܺͺ�5 mol NaOH��ˮ��Һ��ȫ��Ӧ |
| A�� | ��ԭ�ӵĽṹʾ��ͼ��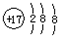 | B�� | �Ȼ�淋ĵ���ʽ��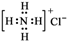 | ||
| C�� | CO2�Ľṹʽ��O=C=O | D�� | CH4�ı���ģ�ͣ� |
| A�� | �û�������C��Hԭ����֮��Ϊ1��2 | |
| B�� | ��ȷ�������ʽ | |
| C�� | ����ʽ����ȷ�����������������ȷ�� | |
| D�� | �û������������������� |
| A�� | ú��������Һ���������ڻ�ѧ�仯 | |
| B�� | ʯ�͵��ѻ�������������͵IJ��������� | |
| C�� | ʯ�ͷ���ɻ����ϩ����ϩ | |
| D�� | �����л����Ǵ�ú��������з�������� |

 ��
�� +2nNaOH��n
+2nNaOH��n +��n+1��H2O��
+��n+1��H2O��