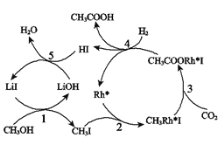
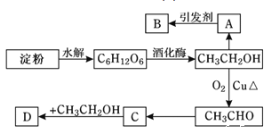
��Ŀ����
����Ŀ����ҵ�кܶ���Ҫ��ԭ�϶�����Դ��ʯ�ͻ������ش���������

��1��A���ṹ��ʽΪ__________________
��2����ϩ�������������ŵ�����Ϊ__________________
��3��д����Ӧ�ٵķ�Ӧ���ͣ�_____________
��4��д��������ͼ��ijЩ���̵Ļ�ѧ����ʽ��
���̢�_________________________
���̢�______________________
���̢�_________________
��5�������й�ʵ���˵������ȷ����_______
A. ��ȥ���������е����ᣬ�ɼ���NaOH��Һ�����÷�Һ
B. �л���C���ϩ������ͬϵ��
C. �۱�ϩ���ܹ�ʹ���Ը��������Һ��ɫ
D. ��ȥ�������л��е�����ŨHNO3��H2SO4���ɽ��䵹�뵽һ������NaOH��Һ�У����÷�Һ
���𰸡�CH2=CH2 �Ȼ� ȡ����Ӧ��������Ӧ CH2=CH2��H2O ![]() CH3CH2OH nCH2=CHCOOH
CH3CH2OH nCH2=CHCOOH![]()
 CH3COOH��CH3CH2OH
CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O D
CH3COOCH2CH3��H2O D
��������
B���ϩ�ᷢ��������Ӧ�����ɱ�ϩ����������BΪCH3CH2OH��A��H2O��Ӧ�����Ҵ�����AΪCH2=CH2��B��C��Ӧ����������������CΪCH3COOH��Ȼ�����������
B���ϩ�ᷢ��������Ӧ�����ɱ�ϩ����������BΪCH3CH2OH��A��H2O��Ӧ�����Ҵ�����AΪCH2=CH2��B��C��Ӧ����������������CΪCH3COOH��
��1����������������A�Ľṹ��ʽΪCH2=CH2��
��2����ϩ��Ľṹ��ʽΪCH2=CHCOOH�����й�������̼̼˫�����Ȼ������к������������Ȼ���
��3����ת����������������ȡ����Ӧ��������Ӧ��
��4�����̢�����ϩ��H2O�����ӳɷ�Ӧ���䷴Ӧ����ʽΪCH2=CH2��H2O ![]() CH3CH2OH�����̢ܱ�ϩ�ᷢ���Ӿ۷�Ӧ���䷴Ӧ����ʽΪnCH2=CHCOOH
CH3CH2OH�����̢ܱ�ϩ�ᷢ���Ӿ۷�Ӧ���䷴Ӧ����ʽΪnCH2=CHCOOH![]()
 �����̢ݷ����ķ�Ӧ��CH3COOH��CH3CH2OH
�����̢ݷ����ķ�Ӧ��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��
��5��A������������NaOH��Һ���ܷ���ˮ�ⷴӦ����˳�ȥ���������е��������NaOH��Һ�����Լ��뱥��̼������Һ�����÷�Һ����A˵������
B���л���CΪCH3COOH�����ϩ�Ậ�еĹ����Ų�ͬ��������߲���Ϊͬϵ���B˵������
C�����ݹ��̢ܵķ�Ӧ����ʽ���۱�ϩ���в���̼̼˫��������ʹ���Ը��������Һ��ɫ����C˵������
D��HNO3��H2SO4��NaOH�����кͷ�Ӧ������NaNO3��Na2SO4����������������ˮ��Һ�壬Ȼ����÷�Һ�ķ������з��룬��D˵����ȷ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�