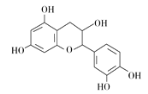
��Ŀ����
����Ŀ��(1)��ϵͳ��������д�����л�������ƣ�![]() ������_____________________��
������_____________________��
(2)д��1,3-����ϩ��������ˮ��Ӧ�ķ���ʽ��_______________________________��
(3)������CH3CH3 ��CH2=CH2 ��CH3CH2C![]() CH ��CH3C
CH ��CH3C![]() CCH3 ��C2H6 ��CH3CH=CH2�У�һ����Ϊͬϵ�����________��һ����Ϊͬ���칹�����(����)________��
CCH3 ��C2H6 ��CH3CH=CH2�У�һ����Ϊͬϵ�����________��һ����Ϊͬ���칹�����(����)________��
(4)ij�л���2.3����ȫȼ�պ�����4.4�˶�����̼��2.7��ˮ����ø��л���������ܶ���2.054g/L(��״��)�����л���ķ���ʽΪ______����д������ܵĽṹ��ʽ������____________________��
���𰸡�3,4-�������� CH2=CH-CH=CH2+2Br2��CH2Br-CHBr-CHBr-CH2Br �ڢ� �ۢ� C2H6O CH3CH2OH�Ҵ���CH3OCH3����
��������
(1)��������ϵͳ������ԭ��������
(2) 1,3-����ϩ��������ˮ�����ӳɷ�Ӧ������1,2,3,4-���嶡�飻
(3)ͬϵ���ǽṹ���ơ��������������ɸ�CH2ԭ���ŵ��л��ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ���л��
(4)���������غ�����л�����C��H��Oԭ�����ȣ��ٸ�����Է�������ȷ�������ʽ�����ݼۼ�������д���ܵĽṹ��ʽ��
(1)��������ϵͳ������ԭ��![]() ������3,4-�������飻
������3,4-�������飻
(2) 1,3-����ϩ��������ˮ�����ӳɷ�Ӧ������1,2,3,4-���嶡�飬��Ӧ����ʽ��CH2=CH-CH=CH2+2Br2��CH2Br-CHBr-CHBr-CH2Br��
(3) C2H6�Ľṹ��ʽ��CH3CH3���٢���ͬһ���ʣ� CH2=CH2��CH3CH=CH2������̼̼˫��������������һ��CH2�����Ԣڢ�����ͬϵ�CH3CH2C![]() CH��CH3C
CH��CH3C![]() CCH3����ʽ��ͬ��̼̼������λ�ò�ͬ�����Ԣܻۢ�Ϊͬ���칹�塣
CCH3����ʽ��ͬ��̼̼������λ�ò�ͬ�����Ԣܻۢ�Ϊͬ���칹�塣
(4) 2.3���л�����ȫȼ�պ����ɵ�CO2�����ʵ�����0.1mol������ˮ�����ʵ�����0.15mol������л�������ԭ�ӵ����ʵ�����![]() ����˸��л�������ʽ��C2H6O�����л���������ܶ���2.054g/L�����Ը��л����Ħ��������2.054g/L��22.4L/mol=46g/mol��������Է���������֪����ʽҲ��C2H6O�����ܵĽṹ��ʽ��CH3CH2OH�Ҵ���CH3OCH3���ѡ�
����˸��л�������ʽ��C2H6O�����л���������ܶ���2.054g/L�����Ը��л����Ħ��������2.054g/L��22.4L/mol=46g/mol��������Է���������֪����ʽҲ��C2H6O�����ܵĽṹ��ʽ��CH3CH2OH�Ҵ���CH3OCH3���ѡ�

 ��״Ԫ���źþ�ϵ�д�
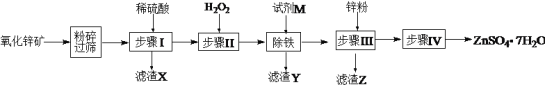
��״Ԫ���źþ�ϵ�д�����Ŀ����ˮ����п����𩷯��������ýȾ������������ľ�ķ���������ҵ��������п��(��Ҫ�ɷ�ΪZnO������ZnSiO3��FeCO3��CuO��)����ZnSO4��7H2O���������£�
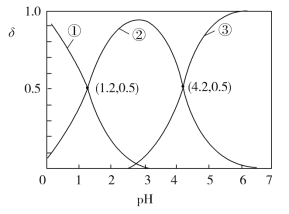
�ڸ������У���������������������pH�����
��ʼ����ʱpH | ��ȫ����ʱpH | |
Zn2+ | 5.4 | 6.4 |
Fe3+ | 1.1 | 3.2 |
Fe2+ | 5.8 | 8.8 |
Cu2+ | 5.6 | 6.4 |
��ش��������⣺
(1)��������п��ʯ��Ŀ����______________������X�ijɷ���________________��
(2)������м���H2O2Ŀ���ǣ�_______________��������Ӧ�����ӷ���ʽΪ��______________��
(3)�������������м����Լ�M������Һ��pH���Լ�M������________(�ѧʽ��һ�ּ���)��������Һ��pH��ΧΪ��_________��ͬʱ����Ҫ����Һ���ȣ���Ŀ���ǣ�__________��
(4)����Z�ijɷ���____________��
(5)ȡ28.70 g ZnSO4��7H2O(��Է���������287)��������ͬ�¶ȣ�ʣ�����������仯��ͼ��ʾ��
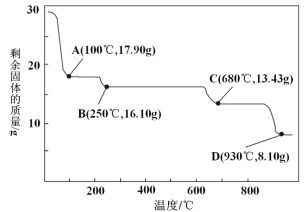
�ٲ�����еĺ�ɲ������ڼ�ѹ�����½��У���ԭ����____________��
��680 ��ʱ���ù���Ļ�ѧʽΪ________________��
a.ZnO b.Zn3O(SO4)2 c.ZnSO4 d.ZnSO4��H2O
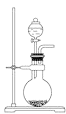
����Ŀ��ijС��ͬѧ����ͼ1װ��(�г�������ʡ��)�Ʊ��屽����̽���÷�Ӧԭ����
��.�Ʊ��屽
(1)װ���г�����a��������__________���������塣
(2)��ʵ������õ��屽Ϊ��ɫ������Ϊ_________.
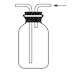
��.�����ᴿ
��֪���屽�뱽���ܣ�Һ�塢�����屽�ķе�����Ϊ59�桢80�桢156�档ͬѧ���������ͼ2���̣�
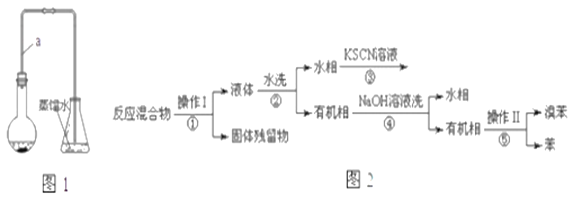
(3)������Ϊ________.
(4)���̢ں͢��У���Ҫ�õ��IJ����������ձ���___�����̢۵�����Ϊ____�����̢ܵ�������_____.
��.̽��ԭ��
(5)��Ӧ��������ijͬѧȡ������ƿ�е�Һ�����Թ������ٵ��뼸��AgNO3��Һ������dz��ɫ�������Ӷ��жϷ�Ӧһ��������HBr������Ϊ��ͬѧ���ж�________(����������������������)��
(6)Ϊ̽����ƿ����Һ����������������̽��ʵ�鷽��(��ѡ�Լ���þ�ۡ����Ȼ�̼����ˮ����ˮ������ˮ)
ʵ�鲽�� | Ԥ������ | ���� |
1.����ƿ�е�Һ��ת���Һ©������������__�����Һ���ֱ�ȡ������_����Һ���Թ�A��B�� | _______ | ______ |
2.���Թ�A�м���������___�����Ȼ�̼�������� | ��Һ�ֲ����²��Ԣ�___ɫ | ��ƿ��Һ�庬����Br |
3.���Թ�B�м����___. | ���������� | ��ƿ��Һ�庬������___ |
�������������ƶϣ��Ʊ��屽�ķ�Ӧ����___��Ӧ���䷴Ӧ����ʽΪ______��