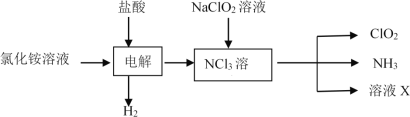
ĚâÄżÄÚČÝ
ˇľĚâÄżˇżÄżÇ°ď®µçłŘµÄÓ¦ÓĂČŐŇćąă·şŁ¬¶ř·ĎľÉ﮵çłŘµÄ»ŘĘŐŔűÓĂĘÇĘ®·ÖÖŘŇŞµÄżÎĚ⡣»ŘĘŐŔűÓĂ·ĎľÉ﮵çłŘµÄÁ÷łĚČçĎÂŁş

ŇŃÖŞŁş˘Ů﮵çłŘ·ĎÁϵÄÖ÷ŇŞłÉ·ÖĘÇLiCoO2ˇ˘ÂÁˇ˘ĚżşÚĽ°ĆäËűÔÓÖʡŁ
˘Úˇ°ČÜŇşAˇ±ÖĐÖ÷ŇŞ˝đĘôŔë×ÓĘÇCo2+ˇ˘Li+Ł¬»ąş¬ÓĐÉŮÁżFe3+ˇ˘Al3+ˇ˘Cu2+ˇŁ
Çë»Ř´đŁş
(1)˛˝Öč˘ńÖĐÂÁČÜ˝âµÄŔë×Ó·˝łĚʽΪ_____Ł»
˛˝Öč˘óÖĐLiCoO2ąĚĚĺČÜ˝âµÄ»ŻŃ§·˝łĚʽΪ_______Ł»
(2)ąŘÓÚ˛˝Öč˘ňŁ¬ĎÂÁĐʵŃé˛Ů×÷»ň˵·¨şĎŔíµÄĘÇ_________
AŁ®×ĆÉŐÇ°Ł¬×ĆÉŐĘąÓõÄŐô·˘ĂóĎ´ľ»şó˛»ĐčŇŞ˛Á¸ÉŁ¬Č»şóĽÓČëąĚĚĺX˝řĐĐ×ĆÉŐ
BŁ®×ĆÉŐʱĐčŇŞÓòŁÁ§°ô˛»¶Ď˝Á°č
CŁ®×ĆÉŐÖÁşăÖŘĘÇָǰşóÁ˝´ÎłĆÁżËůµĂÖĘÁżÖ®˛î˛»µĂł¬ąýŇ»¶¨µÄÔĘĐíÎó˛îŁ¬Őâ¸öÔĘĐíÎó˛îŇ»¶¨ÎŞ0.01g
DŁ®ÔÚµç˝âČŰČÚµÄAl2O3ÖƱ¸˝đĘôÂÁʱŁ¬Í¨łŁĐčĽÓČë±ůľ§ĘŻ(NaAlF6)ŇÔÔöÇżĆ䵼µçĐÔ
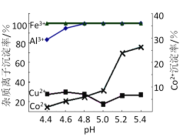
(3)˛˝Öč˘ôÖĐłýÔÓʱżÉĽÓČ백ˮµ÷˝ÚČÜŇşµÄpHŁ¬ĘµŃé±íĂ÷ČÜŇşAÖи÷ÖÖ˝đĘôŔë×ӵijÁµíÂĘËćpHµÄ±ä»ŻČçĎÂÍĽËůĘľŁ¬Ôň˛˝Öč˘ôÖпɳýČĄµÄÔÓÖĘŔë×ÓĘÇ_________ˇŁ
(4)˛˝Öč˘őżÉ˛ÉÓĂËáĽîµÎ¶¨·¨˛â¶¨ĚĽËá﮵Ĵż¶ČˇŁµÎ¶¨ÔŔíČçĎÂŁşĚĽËáď®ÄÜą»ÓëŃÎËá·´Ó¦ÉúłÉÂČ»Żď®şÍ¶ţŃő»ŻĚĽŁ¬ÔÚĚĽËáď®Î´ÍęČ«·´Ó¦Ę±Ł¬ČÜŇş±ŁłÖÖĐĐÔ(pH=7)ˇŁ·´Ó¦ÍęČ«şóŁ¬Ëć×ĹŃÎËáµÄĽĚĐřµÎČ룬ČÜŇşpHĎ½µŁ¬ŇԼ׻ůşěˇŞäĺĽ×·ŰÂĚΪָʾĽÁŁ¬ÓĂŃÎËá±ę׼ҺµÎ¶¨ĘÔŃůŁ¬ÓĂĎűşÄŃÎËá±ę׼µÎ¶¨ČÜŇşµÄÁżŔ´ĽĆËăĚĽËá﮵ĺ¬ÁżˇŁ
˘ŮĹäÖĆÔĽ0.30molL-1ŃÎËáČÜŇş˛»ĐčŇŞÓõ˝µÄŇÇĆ÷ÓĐ________(Ěî±ŕşĹ)
a.ÁżÍ˛b.µç×ÓĚěĆ˝c.©¶·d.ÉŐ±e.ČÝÁżĆżf.˛ŁÁ§°ôg.˝şÍ·µÎąÜ
˘ÚÓĂ0.3000molL-1ŃÎËá±ę׼ČÜŇşµÎ¶¨Ł¬ĆäÖĐŐýČ·˛Ů×÷˛˝ÖčµÄËłĐňÎŞ________
aŁ®ĽÓČë0.1-0.2mLĽ×»ůşěˇŞäĺĽ×·ŰÂĚ×÷ָʾĽÁŁ»
bŁ®Öó·ĐČĄłýCO2Ł¬ÔŮŔäČ´µ˝ĘŇÎÂŁ»
cŁ®˝«ĘÔŃůÖĂÓÚ250mL׶ĐÎĆżÖĐŁ¬ĽÓČë20mLË®Čܽ⣻
dŁ®ÓĂŃÎËá±ę׼ҺµÎ¶¨ÖÁĘÔŇşÓÉÂĚÉ«±äłÉľĆşěÉ«Ł»
eŁ®ĽĚĐřµÎ¶¨ÖÁľĆşěÉ«(µÎ¶¨Í»ÔľÇřÓň)Ľ´ÎŞÖŐµă
˘ŰĚĽËá﮵Ĵż¶ČżÉÓĂĎÂĘ˝ĽĆË㣺¦Ř=![]() Ł¬ĆäÖĐŁş¦ŘˇŞĚĽËáď®ĘÔŃůµÄ´ż¶ČŁ»mˇŞĚĽËáď®ĘÔŃůµÄÖĘÁż(g)Ł»cˇŞŃÎËá±ę׼ҺµÄŨ¶Č(molL-1)V1ˇŞĘÔŃůµÎ¶¨Ę±ĎűşÄŃÎËáµÄĚĺ»ý(mL)Ł»V0ˇŞµÎ¶¨żŐ°×ČÜŇş(Ö¸˛»ĽÓĘÔŃů˝řĐеζ¨)ʱ±ę׼ŃÎËáĎűşÄµÄĚĺ»ý(mL)Ł¬0.03694ˇŞÓë1.00mL±ę׼ŃÎËá(c=1.000molL-1)Ďŕµ±µÄĚĽËá﮵ÄÖĘÁż(g)ˇŁ
Ł¬ĆäÖĐŁş¦ŘˇŞĚĽËáď®ĘÔŃůµÄ´ż¶ČŁ»mˇŞĚĽËáď®ĘÔŃůµÄÖĘÁż(g)Ł»cˇŞŃÎËá±ę׼ҺµÄŨ¶Č(molL-1)V1ˇŞĘÔŃůµÎ¶¨Ę±ĎűşÄŃÎËáµÄĚĺ»ý(mL)Ł»V0ˇŞµÎ¶¨żŐ°×ČÜŇş(Ö¸˛»ĽÓĘÔŃů˝řĐеζ¨)ʱ±ę׼ŃÎËáĎűşÄµÄĚĺ»ý(mL)Ł¬0.03694ˇŞÓë1.00mL±ę׼ŃÎËá(c=1.000molL-1)Ďŕµ±µÄĚĽËá﮵ÄÖĘÁż(g)ˇŁ
µÎ¶¨żŐ°×ČÜŇşµÄÄżµÄĘÇ_________Ł¬ÉĎĘöµÎ¶¨˛Ů×÷ÖĐŁ¬Č±É١°Öó·ĐČĄłýCO2Ł¬ÔŮŔäČ´µ˝ĘŇΡ±Őâ¸ö˛˝Ö裬˛â¶¨˝áąű˝«_________(Ěƫ´óˇ±ˇ˘ˇ°Ć«Đˇˇ±ˇ˘ˇ°ÎŢÓ°Ď족)ˇŁ
ˇľ´đ°¸ˇż2Al+2OH-+2H2OŁ˝2![]() +3H2ˇü»ň 2Al+2OH-+6H2O==2[Al(OH)4]- +3H2ˇü 2LiCoO2+H2O2+3H2SO4Ł˝Li2SO4+2CoSO4+O2ˇü+4H2O B Fe3+ˇ˘Al3+ bc cadbe ŇÔĎűłýʵŃéÖеÄĘÔĽÁˇ˘˛Ů×÷µČ×ŰşĎŇňËŘÔěłÉµÄµÎ¶¨Îó˛î ƫС
+3H2ˇü»ň 2Al+2OH-+6H2O==2[Al(OH)4]- +3H2ˇü 2LiCoO2+H2O2+3H2SO4Ł˝Li2SO4+2CoSO4+O2ˇü+4H2O B Fe3+ˇ˘Al3+ bc cadbe ŇÔĎűłýʵŃéÖеÄĘÔĽÁˇ˘˛Ů×÷µČ×ŰşĎŇňËŘÔěłÉµÄµÎ¶¨Îó˛î ƫС
ˇľ˝âÎöˇż
﮵çłŘ·ĎÁĎÖĐş¬ÓĐLiCoO2ˇ˘ÂÁˇ˘ĚżşÚŁ¬˝«·ĎÁĎĎČÓĂĽîŇş˝ţĹÝŁ¬˝«Alłä·ÖČܽ⣬ąýÂËşóµĂµ˝µÄÂËŇşÖĐş¬ÓĐĆ«ÂÁËáÄĆŁ¬ÔÚ˝ţłöŇşÖĐĽÓČëϡÁňËáÉúłÉÇâŃő»ŻÂÁŁ¬×ĆÉŐˇ˘µç˝âżÉµĂµ˝ÂÁŁ¬ÂËÔüÖĐş¬ÓĐLiCoO2Ł¬˝«ÂËÔüÓĂË«ŃőË®ˇ˘ÁňËá´¦ŔíşóÉúłÉLi2SO4ˇ˘CoSO4Ł¬·´Ó¦µÄŔë×Ó·˝łĚʽΪŁş2LiCoO2+H2O2+3H2SO4=Li2SO4+2CoSO4+O2ˇü+4H2OŁ¬Ěâ¸řĐĹϢżÉÖŞAČÜŇşÖ÷ŇŞµÄ˝đĘôŔë×ÓĘÇCo2+ˇ˘Li+Ł¬»ąş¬ÓĐÉŮÁżFe3+ˇ˘Al3+ˇ˘Cu2+Ł¬ľłýÔÓşóĽÓČë˛ÝËá泥¬żÉµĂµ˝CoC2O4ąĚĚ壬ĸҺÖĐş¬ÓĐLi+Ł¬ĽÓČ뱥şÍĚĽËáÄĆČÜŇşşóąýÂËŁ¬×îşóµĂµ˝ĚĽËá﮹ĚĚ壬ŇÔ´Ë˝â´đ¸ĂĚ⡣
(1)˛˝Öč˘ńÖĐŁ¬ÂÁÓëÇâŃő»ŻÄĆ·´Ó¦ÉúłÉĆ«ÂÁËáÄĆŁ¬Ŕë×Ó·˝łĚʽΪ2Al+2OH-+2H2OŁ˝2![]() +3H2ˇü»ň2Al+2OH-+6H2O==2[Al(OH)4]-+3H2ˇüŁ»˛˝Öč˘óÖĐŁ¬·´Ó¦ÎďÓĐLiCoO2ˇ˘Ë«ŃőË®ˇ˘ÁňËᣬÉúłÉÎďÓĐLi2SO4şÍCoSO4Ł¬LiCoO2ÖĐCo»ŻşĎĽŰ˝µµÍŁ¬ÔňH2O2»ŻşĎĽŰÉý¸ßÉúłÉO2Ł¬ËůŇÔ»ŻŃ§·˝łĚʽΪ2LiCoO2+H2O2+3H2SO4Ł˝Li2SO4+2CoSO4+O2ˇü+4H2OŁ»
+3H2ˇü»ň2Al+2OH-+6H2O==2[Al(OH)4]-+3H2ˇüŁ»˛˝Öč˘óÖĐŁ¬·´Ó¦ÎďÓĐLiCoO2ˇ˘Ë«ŃőË®ˇ˘ÁňËᣬÉúłÉÎďÓĐLi2SO4şÍCoSO4Ł¬LiCoO2ÖĐCo»ŻşĎĽŰ˝µµÍŁ¬ÔňH2O2»ŻşĎĽŰÉý¸ßÉúłÉO2Ł¬ËůŇÔ»ŻŃ§·˝łĚʽΪ2LiCoO2+H2O2+3H2SO4Ł˝Li2SO4+2CoSO4+O2ˇü+4H2OŁ»
(2)˛˝Öč˘ň˝«ÇâŃő»ŻÂÁŁ¬×ĆÉŐˇ˘µç˝âşóµĂµ˝ÂÁŁ¬
AŁ®Őô·˘ĂóŇ»°ăÓĂÓÚŐô·˘ČÜŇşŁ¬ÇâŃő»ŻÂÁÎŞąĚĚ壬һ°ăÓĂŰáŰö×ĆÉŐŁ¬A´íÎóŁ»
BŁ®×ĆÉŐʱĐčŇŞÓòŁÁ§°ô˛»¶Ď˝Á°čŁ¬±ÜĂâľÖ˛żąýČČŁ¬ŇýĆđ±Ä˝¦Ł¬BŐýČ·Ł»
CŁ®ÔÚÖŘÁż·ÖÎö·¨ÖĐŁ¬ľşć¸É»ň×ĆÉŐµÄŰáŰö»ňłÁµíŁ¬Ç°şóÁ˝´ÎłĆÖŘÖ®˛îСÓÚ0.2mgŁ¬C´íÎóŁ»
DŁ®ÔÚµç˝âČŰČÚµÄAl2O3ÖƱ¸˝đĘôÂÁʱŁ¬Í¨łŁĐčĽÓČë±ůľ§ĘŻ(NaAlF6)Ł¬ÄżµÄĘÇ˝µµÍŃő»ŻÂÁµÄČ۵㣬D´íÎóˇŁ
´đ°¸ŃˇBŁ»
(3)ČçÍĽżÉÖŞpH<5.0ʱŁ¬Co2+˛»ÉúłÉłÁµíŁ¬¶řFe3+ˇ˘Al3+Ň×ÉúłÉłÁµí¶řłýČĄŁ¬ËůŇԿɳýČĄµÄÔÓÖĘŔë×ÓĘÇFe3+ˇ˘Al3+Ł»
(4)˘ŮĹäÖĆÔĽ0.30molL-1ŃÎËáČÜŇşĐčŇŞµÄŇÇĆ÷ÓĐÁżÍ˛ˇ˘ÉŐ±ˇ˘ČÝÁżĆżˇ˘˛ŁÁ§°ôˇ˘˝şÍ·µÎąÜŁ¬Ôň˛»ĐčŇŞÓõ˝µÄŇÇĆ÷ÓĐbcŁ»
˘ÚÓĂ0.3000molL-1ŃÎËá±ę׼ČÜŇşµÎ¶¨Ł¬´óĚ岽ÖčÎŞŁşČˇŃůÓÚ׶ĐÎĆżÖĐŁ¬ĽÓË®Čܽ⣻ĽÓČëָʾĽÁŁ»ÓĂŃÎËá±ę׼ҺµÎ¶¨ÖÁָʾĽÁ±äÉ«Ł»Öó·ĐČĄłýCO2Ł¬ÔŮŔäČ´Ł»ĽĚĐřµÎ¶¨ÖÁָʾĽÁ±äÉ«Ľ´Î޵ζ¨Öյ㣬ËůŇÔËłĐňÎŞcadbeŁ»
˘ŰµÎ¶¨żŐ°×ČÜŇşµÄÄżµÄĘÇĎűłýʵŃéÖеÄĘÔĽÁˇ˘˛Ů×÷µČ×ŰşĎŇňËŘÔěłÉµÄµÎ¶¨Îó˛îŁ»ÉĎĘöµÎ¶¨˛Ů×÷ÖĐŁ¬ČçȱÉŮÖó·ĐČĄłýCO2Ł¬ÔňCO2ČÜÓÚˮʹČÜŇşłĘËáĐÔŁ¬ËůĽÓŃÎËáĚĺ»ýĆ«ÉŮŁ¬ËůŇԲⶨ˝áąű˝«Ć«ĐˇˇŁ

ˇľĚâÄżˇżÔÚ1LşăČÝĂܱŐČÝĆ÷ÖĐłäČëX(g)şÍY(g)Ł¬·˘Éú·´Ó¦X(g)+Y(g)![]() M(g)+N(g)Ł¬ËůµĂʵŃéĘýľÝČçĎÂ±íŁş
M(g)+N(g)Ł¬ËůµĂʵŃéĘýľÝČçĎÂ±íŁş
ʵŃé±ŕşĹ | ζČ/ˇć | ĆđʼʱÎďÖʵÄÁż/mol | Ć˝şâʱÎďÖʵÄÁż/mol | |
n(X) | n(Y) | n(M) | ||
˘Ů | 700 | 0.10 | 0.10 | 0.09 |
˘Ú | 800 | 0.20 | 0.20 | 0.10 |
˘Ű | 800 | 0.20 | 0. 30 | a |
˘Ü | 900 | 0.10 | 0.15 | b |
ĎÂÁĐ˵·¨´íÎóµÄĘÇ
A. ʵŃé˘ŮÖĐŁ¬Čô5minʱ˛âµĂn(M) =0.05molŁ¬Ôň0ÖÁ5minʱĽäÄÚŁ¬ÓĂN±íĘľµÄĆ˝ľů·´Ó¦ËŮÂĘv(N) =0.01 mol/( Lˇ¤min)
B. ʵŃé˘ÚÖĐŁ¬¸Ă·´Ó¦µÄĆ˝şâłŁĘýK= 1.0
C. ʵŃé˘ŰÖĐŁ¬´ďµ˝Ć˝şâʱŁ¬XµÄת»ŻÂĘÎŞ60%
D. ʵŃé˘ÜÖĐŁ¬´ďµ˝Ć˝şâʱŁ¬b>0.06
ˇľĚâÄżˇż(I)ŇŃÖŞÔÚ448 ˇćʱŁ¬·´Ó¦H2(g)Ł«I2(g)![]() 2HI(g)µÄĆ˝şâłŁĘýK1ÎŞ49Ł¬Ôň¸ĂζČĎ·´Ó¦2HI(g)
2HI(g)µÄĆ˝şâłŁĘýK1ÎŞ49Ł¬Ôň¸ĂζČĎ·´Ó¦2HI(g)![]() H2(g)Ł«I2(g)µÄĆ˝şâłŁĘýK2ÎŞ______Ł»·´Ó¦
H2(g)Ł«I2(g)µÄĆ˝şâłŁĘýK2ÎŞ______Ł»·´Ó¦![]() H2(g)+
H2(g)+![]() I2(g)
I2(g)![]() HI(g)µÄĆ˝şâłŁĘýK3ÎŞ________ˇŁ
HI(g)µÄĆ˝şâłŁĘýK3ÎŞ________ˇŁ
(II)ÔÚŇ»¶¨Ěĺ»ýµÄĂܱŐČÝĆ÷ÖĐ˝řĐĐ»ŻŃ§·´Ó¦ŁşCO2(g)Ł«H2(g)![]() CO(g)Ł«H2O(g)Ł¬Ć仯ѧƽşâłŁĘýşÍζȵĹŘϵČçϱíËůĘľŁş
CO(g)Ł«H2O(g)Ł¬Ć仯ѧƽşâłŁĘýşÍζȵĹŘϵČçϱíËůĘľŁş
t/ˇć | 700 | 800 | 830 | 1 000 | 1 200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
»Ř´đĎÂÁĐÎĘĚ⣺
(1)¸Ă·´Ó¦µÄ»ŻŃ§Ć˝şâłŁĘý±í´ďʽΪK=_____ˇŁ
(2)¸Ă·´Ó¦ÎŞ_____(ĚÎüČȡ±»ňˇ°·ĹČȡ±)·´Ó¦ˇŁ
(3)ijζČĎÂŁ¬Ć˝şâŨ¶Č·űşĎĎÂĘ˝Łşc(CO2)ˇ¤c(H2)=c(CO)ˇ¤c(H2O)Ł¬ĘÔĹжϴËʱµÄζČÎŞ_____ˇćˇŁ
(4)ÔÚ800ˇćʱŁ¬·˘ÉúÉĎĘö·´Ó¦Ł¬ÄłŇ»Ę±żĚ˛âµĂČÝĆ÷ÄÚ¸÷ÎďÖʵÄŨ¶Č·Ö±đÎŞc(CO2)=2molˇ¤L1Ł¬c(H2)=1.5molˇ¤L1Ł¬c(CO)=1molˇ¤L1Ł¬c(H2O)=3molˇ¤L1Ł¬ÔňĎÂһʱżĚŁ¬·´Ó¦Ďň_____(ĚŐýĎňˇ±»ňˇ°ÄćĎňˇ±)˝řĐСŁ