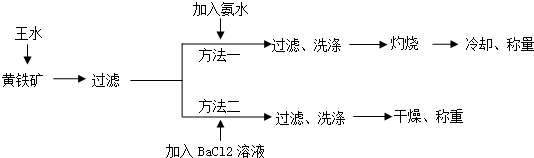
��Ŀ����
12��������ʵ������йص��ǣ���������NH3���ۡ��е�Ȣ�A������Ԫ���⻯����ۡ��е��
��I2������CCl4
��HF��HCl��HBr��HI�����ȶ������μ���
�����ǻ���������ۡ��е�ȶԼ���������ۡ��е��
��ˮ���ȵ��ܸߵ��¶ȶ����Էֽ�
��CH4��SiH4��GeH4��SnH4�۵�����Է����������������
��ˮ��ɱ�������ͣ�
| A�� | �٢ڢܢޢ� | B�� | �٢ܢݢ� | C�� | �٢ܢ� | D�� | �ڢۢ� |
���� �٢�A���У�N�ķǽ�������ǿ��NH3�з���֮����������
��I2������CCl4��������������ԭ����
��HF��HCI��HBr��HI�����ȶ������μ�������±�صķǽ������йأ�
�ܶ��ǻ����������γɷ���֮�������
���ȶ����뻯ѧ���йأ�
��CH4��SiH4��GeH4��SnH4�۵�����Է����������Ӷ����ߣ�������أ�
�߱��д����������������
��� �⣺����ڢ٢�A���У�N�ķǽ�������ǿ��NH3�з���֮������������NH3���ۡ��е�Ȣ�A������Ԫ���⻯��ĸߣ��ʢ���ȷ��
��I2������CCl4��������������ԭ����������أ��ʢڴ���
��HF��HCl��HBr��HI�����ȶ������μ�������±�صķǽ������йأ�������أ��ʢ۴���
�ܶ��ǻ����������γɷ���֮������������ǻ��������γɷ�����������������ǻ���������ۡ��е�ȶ��ǻ�������ĵͣ��ʢ���ȷ��
��ˮ���ȵ��ܸߵ��¶ȶ����Էֽ⣬���ȶ����뻯ѧ���йأ���������أ��ʢݴ���
��CH4��SiH4��GeH4��SnH4�۵�����Է����������Ӷ����ߣ����ӽṹ���Ƶģ���Է�������Խ���۵�Խ�ߣ�������أ��ʢ���
�߱��д��������������������ͬ����ʱ�����ܶȱ�Һ̬ˮ���ܶ�С���ʢ���ȷ��
��ѡC��
���� ���⿼���������������ʵ����ʵ�Ӱ�죬��ȷ�����ҪӰ�����ʵ����������ǽ����Ĺؼ�����Ŀ�ѶȲ���

| A�� | CuS | B�� | FeCl2 | C�� | FeS | D�� | SO3 |
| A�� | �е�ߵͣ�HI��HBr��HCl��HF | |
| B�� | ���ȶ��Դ�С��HF��H2O��NH3��PH3 | |
| C�� | �۵�ߵͣ����ʯ��ʳ�Σ������ƣ��� | |
| D�� | ���뾶��С��S2-��Cl-��F-��Na+��Al3+ |
| A�� | 1L 1mol•L-1����������Һ�к��е�Al3+Ϊ2NA | |
| B�� | pH=1��H2SO4��Һ�к���H+����ĿΪ0.2NA | |
| C�� | ������5.6 g Fe��������Ũ���ᷴӦ��ת�Ƶ��ӵ���ĿΪ0.3NA | |
| D�� | 27g���������������NaOH��Һ��Ӧ��ת�Ƶ��ӵ���Ŀ��Ϊ3NA |
| A�� | �������6��ԭ�Ӵ���ͬһƽ�� | |
| B�� | �����������12��ԭ�Ӵ���ͬһƽ���� | |
| C�� | ����ϩ�����16��ԭ�Ӵ���ͬһƽ���� | |
| D�� | CH3CH=CH-C��C-CF3�����10��ԭ�Ӵ���ͬһƽ���� |
| A�� | �л��ﲻһ����������ˮ | |
| B�� | �л��ﶼ�ǹ��ۻ����� | |
| C�� | �л��ﶼ�Ǵ��л����з������������ | |
| D�� | �л��ﲻ�߱���������� |
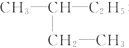 ��
��
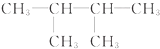 ��
��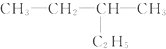
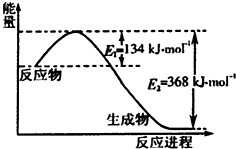 ̼�ǵ����Ϻ����ḻ��Ԫ�أ�����������о�������Ҫ���壮
̼�ǵ����Ϻ����ḻ��Ԫ�أ�����������о�������Ҫ���壮