��Ŀ����
1����ʯ�������Ѿ������е�һ���л��ᣬ���ѾƵ�pH��Ҫȡ���ھ�ʯ��ĺ��������������Ѿ�pHԼΪ2.9��3.8�������£���ʯ�ᣨ��H2T��ʾ��ˮ��Һ����������ռ�ķ�����a����pH�Ĺ�ϵ��ͼ��ʾ�����б�������ȷ���ǣ�������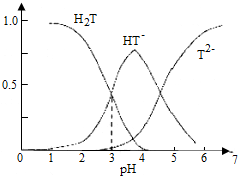
| A�� | ���Ѿ��г��˴��ھ�ʯ���⣬�����ھ�ʯ���� | |
| B�� | �����£�H2T?H++HT- Ka=10-3 | |
| C�� | �����ѾƵ�pHΪ3.7ʱ��HT-��ռ�����ﵽ���ʱ���Ѿ���H2T��T2- | |
| D�� | ��HT-��T2-���ʵ���Ũ�����ʱ����Һ��ˮ���������������Ũ��С�ڴ�ˮ�������ӵ�Ũ�� |
���� A����ͼ���֪������Һ��pHԼΪ2.9��3.8����Һ��ҪΪHT-��
B������H2T?H++HT-��Ka=$\frac{c��H{\;}^{+}��c��HT{\;}^{-}��}{c��H{\;}_{2}T��}$���м��㣻
C����ͼ���֪�������ѾƵ�pHΪ3.7ʱ��HT-��ռ�����ﵽ���ʱ���Ѿ���H2T��T2-�ķ�����ͬ��
D����ͼ���֪����HT-��T2-���ʵ���Ũ�����ʱ��pHΪ3.7ʱ������ˮ�ĵ��룬�ݴ˷�����
��� �⣺A����ͼ���֪������Һ��pHԼΪ2.9��3.8����Һ��ҪΪHT-���������Ѿ��г��˴��ھ�ʯ���⣬�����ھ�ʯ���Σ���A��ȷ��
B����ΪK�����¶��йأ�����ȡ��ҺpHΪ3ʱ����HT-��H2T���ʵ���Ũ����ȣ���H2T?H++HT-��Ka=$\frac{c��H{\;}^{+}��c��HT{\;}^{-}��}{c��H{\;}_{2}T��}$=c��H+��=10-3����B��ȷ��
C����ͼ���֪�������ѾƵ�pHΪ3.7ʱ��HT-��ռ�����ﵽ���ʱ���Ѿ���H2T=T2-����C����
D����ͼ���֪����HT-��T2-���ʵ���Ũ�����ʱ��pHΪ3.7ʱ������ˮ�ĵ��룬���Դ�ʱ��Һ��ˮ���������������Ũ��С�ڴ�ˮ�������ӵ�Ũ�ȣ���D��ȷ��
��ѡC��
���� ������ͼ�����ʽ����ƽ�ⳣ���ļ��㡢���ӱȽϡ�Ӱ��ˮ�ĵ������صȣ���Ŀ�Ѷ��еȣ���Ҫѧ��������ʵ�Ļ������ͼ��ȡ��Ϣ��������

| Ԫ�� | �����Ϣ |
| X | X������������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
| Y | Y�ǵؿ��к�����ߵ�Ԫ�� |
| Z | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p1 |
| W | W��һ�ֺ��ص�������Ϊ28��������Ϊ14 |
��2��Z�ĵ�һ�����ܱ�W��С�����С������ XY2�ɹ�̬��Ϊ��̬����˷��������������Ƿ��Ӽ�����������Ԫ�ء�X��Y��ԭ�ӿɹ�ͬ�γɶ��ַ��ӣ�д������һ�����γ�ͬ�ַ��Ӽ��������������CH3CH2OH��CH3COOH�ȣ�
��3��W�ĵ���������ᷴӦ����������ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽ��Si+4HF=SiF4��+2H2����
| A�� | �����£�1molͭƬͶ�뺬��4mol HNO3��Ũ�����У�ͭƬ������ȫ�ܽ� | |
| B�� | Al��Sֱ�ӻ��Ͽ��Եõ�Al2S3��Fe��Sֱ�ӻ���Ҳ���Եõ�Fe2S3 | |
| C�� | ���AlCl3��FeCl3��CuCl2�Ļ����Һ�У���������������Cu��Fe��Al | |
| D�� | ��Ӧ14CuSO4+5FeS2+12H2O=7Cu2S+5FeSO4+12H2SO4��FeS2�е���Ԫ��ȫ�������� |
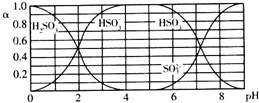
| A�� | ��Һ��pH=5ʱ����Ԫ�ص���Ҫ������ʽΪHSO3- | |
| B�� | ����Һǡ�ó�����ʱ��c��Na+����c��SO32-��+c��HSO3-�� | |
| C�� | ��pH=8��������Һ�еμ���������ʯ��ˮ��$\frac{{c��{HSO_3^-}��}}{{c��{SO_3^{2-}}��}}$��ֵ���� | |
| D�� | ��pH=3��������Һ�еμ�����ϡ���ᣬ����HSO3-����С |
| A�� |  ��ȡ�ռ����ﰱ�� | B�� |  ̼���������ȷֽ� | ||
| C�� |  ��ȥCO�����е�CO2���� | D�� | 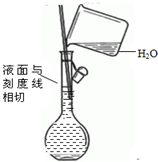 ������Һ |
| A�� | 52.2% | B�� | 31.4% | C�� | 30% | D�� | 27.5% |
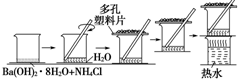 �о���ѧ��Ӧ�е������仯ʱ����һֻС�ձ������20g����ĥ�ɷ�ĩ��Ba��OH��2•8H2O����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ�����ձ��м���Լ10g NH4Cl���壬������ͼ��ʾʵ�鲽����в������ش��������⣺
�о���ѧ��Ӧ�е������仯ʱ����һֻС�ձ������20g����ĥ�ɷ�ĩ��Ba��OH��2•8H2O����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ�����ձ��м���Լ10g NH4Cl���壬������ͼ��ʾʵ�鲽����в������ش��������⣺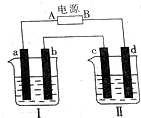 ��ͼ��ʾװ���У��ձ����зֱ�ʢ��200g 9.4%��Cu��NO3��2��Һ�������ı���K2SO3��Һ�����õ缫��Ϊ���Ե缫����ش��������⣺
��ͼ��ʾװ���У��ձ����зֱ�ʢ��200g 9.4%��Cu��NO3��2��Һ�������ı���K2SO3��Һ�����õ缫��Ϊ���Ե缫����ش��������⣺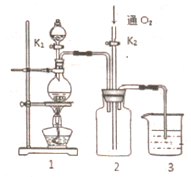 ����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ͭ������ϡ����ֱ�ӷ�Ӧ����ʵ���н�����Ũ����ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���壨װ����ͼ1��2��ʾ��
����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ͭ������ϡ����ֱ�ӷ�Ӧ����ʵ���н�����Ũ����ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���壨װ����ͼ1��2��ʾ��