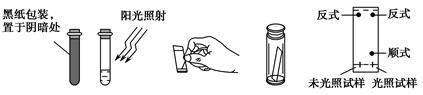
��Ŀ����
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϡ�����ʯ��Ҫ�ɷ�ΪCaCO3�������������ĺ����ʵ�����ô���ʯ��ϡ���ᷴӦ�Ʊ�CO2���塣����װ�ÿ�����CO2������ᴿ���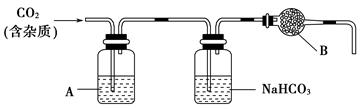
���������գ�
(1)��Ũ��������1��1(�����)��ϡ����(Լ6 mol��L��1)��Ӧѡ�õ�������________(����ĸ����ͬ)��
a���ձ������� b��������������c����Ͳ������ d������ƿ
(2)����װ���У�A��______��Һ��NaHCO3��Һ��������________________��
(3)����װ���У�B������________�������ʵ��õ�������ⶨCO2�ķ����������B����ʧЧ���ⶨ���________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
(4)һ���Է�����ʯ��(������)��CaCO3��ʳ���е��ܳ��������۷���������ָ��֮һ���ⶨ�ܳ�������Ҫʵ�鲽��������£����顢���ء������ܽ�����ˡ�������ɡ���ȴ�����ء����ء�Ϊ�˽�ʯ����̼����ܳ���Ӧѡ�õ��Լ���________��
a���Ȼ�����Һ���� b��ϡ���ᡡ��c��ϡ���ᡡ�� d��������
(5)���ܳ����ⶨʵ���У�Ϊ�˻��ʯ����̼��Ƶ�����ܳ�����Ӧ���ܳ�________�����ܳ�________��
(6)�����ⶨʵ���У�����________��˵����Ʒ�Ѿ����ء�
��(1)abc��(2)CuSO4������KMnO4��HCl������������塡(3)��ˮCaCl2��P2O5��ƫ�͡�(4)bd
(5)ʯ����CaCO3
(6)���γ����Ľ��������(��С��)0.1 g
����

������������C9H10O2������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��
��֪��
| | ��ɫ��״̬ | �е㣨�棩 | �� �ܶȣ�g��cm��3�� |
| *������ | ��ɫ��Ƭ״���� | 249 | 1.2659 |
| ���������� | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | ��ɫ����Һ�� | 80.8 | 0.7318 |
*��������100 ���Ѹ��������
ʵ�鲽�����£�
a����100 mLԲ����ƿ�м���12.20 g�����ᡢ25 mL�Ҵ�����������20 mL �����飬�Լ�4 mLŨ���ᣬ��Ͼ��Ȳ������ʯ������ͼ��ʾװ�������������¶���65��70 ����Ȼ���2 h����Ӧʱ�����顪�Ҵ���ˮ���γɡ���������е�62.6 �棩��������������÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
b����Ӧ�������������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
c������ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������Na2CO3����Һ�����ԡ�
d���÷�Һ©���ֳ��л��㣬ˮ����25 mL������ȡ��Һ��Ȼ��ϲ����л��㡣�����Ȼ��ƣ��Դֲ�������������������Ѻ������£�����210��213�����֡�
e������ϸ�ò�Ʒ���Ϊ12.86 mL��
�ش��������⣺
��1���ٲ���a��ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����__________________________��
�ڲ���b��Ӧ������ֵ��¶���________��
A��65��70 �� B��78��80 �� C��85��90 �� D��215��220 ��
�ۼ����Ҵ���������Ҫԭ����____________________________________��
��2����Na2CO3���벻�㣬�ڲ���d����ʱ��������ƿ�пɼ����������ɣ������������ԭ����_____________________________________________________________________��
��3�����ڲ���d�еķ�Һ����������ȷ����________��
A��ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ�����������Һ©����ת������������ҡ
B����ҡ���κ����Һ©���¿ڵIJ�����������
C����������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D���ų�Һ��ʱ���轫�������ϵİ��۶�©�����ϵ�С��
��4����ʵ��IJ���Ϊ________��
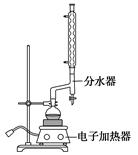
��ͼ1��ʾ��ʵ��������ȡ�����һ�ּ���װ�á�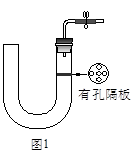
��1��������������������Եķ���_______________________��
��2��������ͼ1��ʾװ�ÿ�����ȡ���Ӧ��״����������Ӧ�Ƿ���Ҫ��������________________________���塣
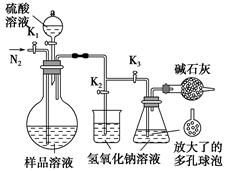
ijͬѧ�����ͼ2��ʾװ�ã��ô�������16.9%ϡ���ᷴӦ��ȡNO���岢̽�����������ļ�̬����ش��й����⡣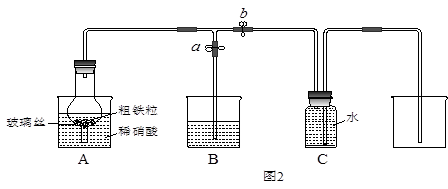
��3����֪16.9%ϡ������ܶ�Ϊ1.10g/cm3���������ʵ���Ũ��Ϊ____________��������������λС������������63%����������16.9%ϡ����500mL������IJ��������в��������ձ��� ��
��4������ֹˮ��a���ر�ֹˮ��bʱ��Aװ�õĸ�����й۲쵽��������_______________________��Bװ���ձ���Һ���������__________________________________����Aװ�������弸����ɫʱ����ֹˮ��b���ر�ֹˮ��a������Cװ���ռ�NO���塣
��5����֪���з�Ӧ���Է�����Fe2O3+3KNO3+4KOH 2K2FeO4+3KNO2+2H2O����Aװ���е�ϡ���ἴʹ����Ũ���ᣬҲ��������+6�۵����Ļ������ԭ����________��
2K2FeO4+3KNO2+2H2O����Aװ���е�ϡ���ἴʹ����Ũ���ᣬҲ��������+6�۵����Ļ������ԭ����________��
a��HNO3�������Ա�KNO3��
b����Ӧ���¶Ȳ���
c��HNO3�����ȶ��Ա�KNO3��
d��FeO42�����ܴ�����������Һ��
��6������������ҩƷ���Թܺͽ�ͷ�ιܣ�0.1mol/LKSCN��Һ��0.2mol/L����KMnO4��Һ��0.1mol/LKI��Һ����ˮ�ȡ��������һ����ʵ�飬̽��Aװ���ձ�����ȫ��Ӧ�������ܵļ�̬����д����ʵ�鱨�棺
| ʵ�鲽�� | ���� | ��������� |
| ��һ�� | ȡ����Һ��װ���Թܣ����� ���е��뼸��KSCN��Һ�� | |
| �ڶ��� | | ����Һ��ɫ��ȥ����˵������Fe2+�� �������Ա仯����˵������Fe2+�� |
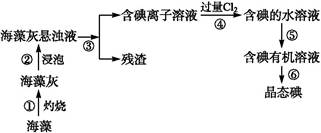
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե�������ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�
��1��ָ����ȡ��Ĺ������й�ʵ����������ƣ�
��Ϊ ����Ϊ �����̢����йط�Ӧ�����ӷ���ʽ�� ��
��2����ȡ��Ĺ����пɹ�ѡ����л��ܼ���( )
| A���ױ����ƾ� | B�����Ȼ�̼���� | C�����͡����� | D�����͡����� |
��4���Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�������Ҫ��������ָ����ͼ��ʾ��ʵ��װ���еĴ���֮���� �� ���� ��
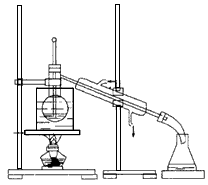
��5�����������������ʱ��ʹ��ˮԡ���ȵ�ԭ���� �����̬���� ��ۼ���
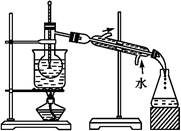

 ����,�������ϵ�֪һ���ϳ�·��:
����,�������ϵ�֪һ���ϳ�·��: CH2+CO+H2
CH2+CO+H2 CH3CH2CH2CHO
CH3CH2CH2CHO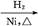 CH3CH2CH2CH2OH;
CH3CH2CH2CH2OH; CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
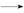 RCH(OH)SO3Na��;�ڷе�:����34��,1
RCH(OH)SO3Na��;�ڷе�:����34��,1 ��Һ
��Һ
 ���
��� 1
1 ��Ʒ
��Ʒ