��Ŀ����
ijС��ͬѧ��һ��Ũ��NaHCO3��Һ���뵽CuSO4��Һ�з��������˳�������ͬѧ��Ϊ������CuCO3;��ͬѧ��Ϊ������CuCO3��Cu(OH)2�Ļ����,�������ʵ��ⶨ������CuCO3������������
(1)���ռ�ͬѧ�Ĺ۵�,������Ӧ�����ӷ���ʽΪ ��
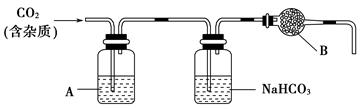
(2)��ͬѧ������ͼ��ʾװ�ý��вⶨ:
�����о����������ǰ,�뽫��������Һ�з��벢�����������������Ϊ���ˡ�ϴ�ӡ����
��װ��E�м�ʯ�ҵ������� ��
��ʵ������������²�������:
a.�ر�K1��K3,��K2��K4,��ַ�Ӧ
b.��K1��K4,�ر�K2��K3,ͨ���������
c.��K1��K3,�ر�K2��K4,ͨ���������
��ȷ��˳���� (��ѡ�����,��ͬ)����δ���в��� ,��ʹ�������ƫ�͡�
����������Ʒ����Ϊm g,װ��D����������n g,�������CuCO3����������Ϊ ��
(3)��ͬѧ��Ϊ������ͨ������CO2����������� ���ⶨ������CuCO3������������
(1)Cu2+ + 2HC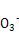 =uCO3��+ CO2��+ H2O
=uCO3��+ CO2��+ H2O
(2)�ڷ�ֹ������CO2��ˮ��������װ��D �� cab b
��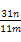 ��100%
��100%
(3)��Ʒ����(����Ʒ������պ������)(����������Ҳ��)
����

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�ʵ��������������ˮ���������Ҵ��Ʊ�1��2�����������װ������ͼ��ʾ��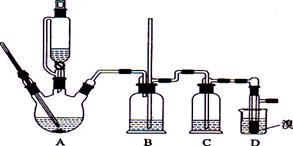
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g �� cm-3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | -l30 | 9 | -1l6 |

�ش��������⣺
��1����ƿD�з�������Ҫ�ķ�Ӧ����ʽ ��
��2����ȫƿB���Է�����,�����Լ��ʵ�����ʱ�Թ�D�Ƿ�����������д����������ʱƿB�е����� ��
��3����װ��C��Ӧ���� (����ĸ) ����Ŀ����_______________��
a��ˮ b��Ũ���� c������������Һ
��4����������������δ��Ӧ��Br2������� ϴ�ӳ�ȥ��������ĸ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��ȡ�������Ҫ���������� ��
��5�������������������������ѣ����� �ķ�����ȥ��
��6����Ӧ������Ӧ����ˮ��ȴװ��D�����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���� ��
��7���жϸ��Ƹ���Ӧ�Ѿ�������������� ��
��������ƣ�Na2S2O3��5H2O�����������մ��ֳ�Ϊ�����������ڷ�֯��ҵ��������֯ƷƯ������ȼ���Ⱦë֯�����Ⱦ��������Ⱦ�ϵķ�����ֽ�����ȼ���ҽҩ��ҵ������ϴ�Ӽ�������������ɫ���ȣ���������ˮ���������Ҵ��������ֽ⡣��ҵ�ϳ����������Ʒ�������Ʊ���ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ�����ͼ��
ʵ������������Ϊ��
�ٿ�����Һ©����ʹ�����������£��ʵ����������У�ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ������Ž�����������
����������������ʧ��������Һ��pH�ӽ�7ʱ��ֹͣͨ��SO2 ���塣
�۳������õ���Һ��ת�����������У�ˮԡ����Ũ����ֱ����Һ������־�Ĥ��
����ȴ�ᾧ�����ˡ�ϴ�ӡ�
�ݽ������������У���40��450C���Ҹ���50��60min����������ش��������⣺
��1������b�������� ��
��2���������������PHֵС��7������ʻ��½����������ӷ���ʽ����ԭ�� ��
��3��������в��ܽ���Һ�������ɵ�ԭ���� ����Ĥͨ������Һ������ֵ�ԭ���� ��
��4���������ϴ����������ƾ����Լ��Ľṹʽ�� ��
��5�������йس��˵�˵���У���ȷ���� ��
A��Ϊ�˼���ϴ���Ƿ���ȫ��Ӧ��������ƿ�밲ȫƿ֮����Ƥ�ܣ�������ƿ�Ͽڵ�������ϴ��Һ���Թ��н������ʵ��
B������ǰ�����ܼ�����ֽʪ��ʹ��ֽ��©���ײ�����
C�����˽���ʱӦ�ȹس����ã���������ƿ�ӹ�
D����ϴ�ӳ���ʱ��Ӧ��Сˮ��ͷ��ʹϴ�Ӽ�����ͨ��������
��6��Ϊ�����ƵõIJ�Ʒ�Ĵ��ȣ���ʵ��С���ȡ5.0�˵IJ�Ʒ���Ƴ�250mL�����������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���25mL 0.01mol/L KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+=3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+S4O62-������ɫ��ȥ�Ұ���Ӳ���ɫʱ����ζ��յ㡣ʵ���������±���
| ʵ����� | 1 | 2 | 3 |
| Na2S2O3��Һ�����mL�� | 19.98 | 20.02 | 21.18 |
��ò�Ʒ�Ĵ����� ����ӵ������ζ������п������ʵ����ƫ�͵��� ��
A���ζ���δ��Na2S2O3��Һ��ϴ
B���ζ��յ�ʱ���Ӷ���
C����ƿ������ˮ��ϴ
D���ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢������
E���ζ�ʱ����ƿ�Ͼ���
��˾ƥ��(����ˮ����, )��������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ�,�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ����(���ǻ�������)�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ��,�Ʊ�����������������:
)��������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ�,�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ����(���ǻ�������)�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ��,�Ʊ�����������������:
������+ˮ����
 �ֲ�Ʒ
�ֲ�Ʒ
 ����ˮ����
����ˮ����
(��˾ƥ��) ���۵�
���۵�
��Ҫ�Լ��Ͳ�Ʒ����������
| ���� | ��Է������� | �۵��е�(��) | ˮ |
| ˮ���� | 138 | 158(�۵�) | �� |
| ������ | 102 | 139.4(�е�) | ��Ӧ |
| ����ˮ���� | 180 | 135(�۵�) | �� |
�����������Ϣ�ش���������:
(1)�Ʊ���˾ƥ��ʱ,Ҫʹ�ø����������ԭ���� ��
(2)�ٺϳɰ�˾ƥ��ʱ,����ʵļ��ȷ������� ��
�ڼ��Ⱥ���ȴ,δ���ֱ���ȴ�������о�������,��ʱӦ��ȡ�Ĵ�ʩ��������������������
�۳������ôֲ�ƷҪ��������ˮϴ��,��ϴ�ӵľ����������������������������������
(3)�Լ�A��������������������
��һ�ָĽ����ᴿ��������:
�ֲ�Ʒ
 ��
�� ��
�� ��
�� ��
�� ����ˮ����
����ˮ����(4)�Ľ����ᴿ�����м��Ȼ�����װ����ͼ��ʾ,
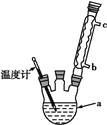
��ʹ���¶ȼƵ�Ŀ������ ��
�ڴ����ᴿ����������������������:�� ,���ò�Ʒ���л�����Ҫ��ԭ������,ԭ������ ��
����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����̽����ʵ�飬�ⶨ��Ӧ������SO2��SO3��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
[�������]
��.��������ijɷֿ���ֻ��SO3һ�֣�
��.��������ijɷֿ��ܺ���________���֣�
��.��������ijɷֿ��ܺ���________���֡�
[ʵ��̽��]
ʵ����������ԡ���֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1��������װ̽��ʵ���װ�ã����������ҵķ��������ӿڵ�����˳��Ϊ�١��������ޡ��ݡ�________��________��________��________���ڣ���ӿ���ţ���
��2����ʵ�����ʱB����Ͳû���ռ���ˮ����֤������________��ȷ��
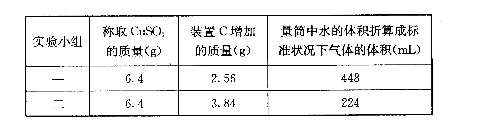
��3��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
| ʵ�� С�� | ��ȡCuSO4 ������/g | װ��C���� ������/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
| һ | 6.4 | 2.56 | 448 |
| �� | 6.4 | 2.56 | 224 |
��ͨ�����㣬�ƶϳ���һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺________________________________________________________��
�ڶ�С�飺________________________________________________________��