��Ŀ����
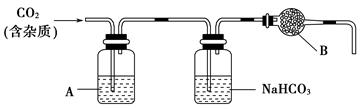
������������Ԫ��֮һ,����ֲ���纣���������к��зḻ�ġ��Ի���̬��ʽ���ڵĵ�Ԫ�ء���ʵ������,�Ӻ�������ȡ������̺�ʵ��װ������: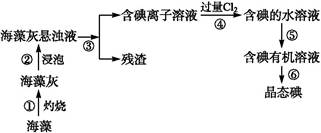
(1)ָ��������ȡ��Ĺ������й�ʵ�����������:�������������,���������������
(2)д������ܶ�Ӧ��Ӧ�����ӷ���ʽ:����������������������
(3)��ȡ��Ĺ�����,�ɹ�ѡ����л��Լ�������������(����)
A.�ƾ� B.���� C.���Ȼ�̼ D.��
(4)����ܳ��˼������Cl2,�������������ѡ����������(�����)��
A.Ũ���� B.H2O2��Һ C.KMnO4��Һ
������____________________________��
(5)Ϊ��ʹ������еĵ�����ת��Ϊ����л���Һ,����ɲ��������,ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ,��ȱ�ٵIJ�������������������
(6)�Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�,����Ҫ��������ָ����ͼʵ��װ���д��ڵĴ���֮��:���� 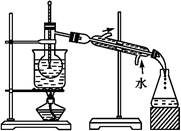
(1)���ˡ���ȡ����Һ(ÿ��1��)
(2)Cl2+2I- I2+2Cl-(2��)
I2+2Cl-(2��)
(3)CD(1��)
(4)B(1��)
������������ɫ������,�����������в�����������,Ҳ��������Ⱦ(2��)
(5)��Һ©������ͨ©��(2��)
(6)�¶ȼ�ˮ�����λ�ò���(2��)
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
CH3CH2OH CH2=CH2+H2O
CH2=CH2+H2O
CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�Ũ������Ҵ�����ΪCO2�ȡ�
������������������Ҵ��Ʊ�1��2-���������װ������ͼ��ʾ��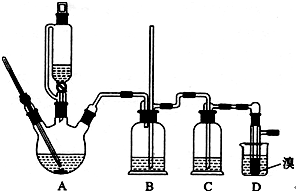
����������:
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm3 | 0��79 | 2��2 | 0��71 |
| �е�/oC | 78��5 | 132 | 34��6 |
| �۵�/oC | -130 | 9 | -116 |
��1��Aװ���Ϸ�ʹ�õ�Һ©�����ŵ��ǣ�_________________________���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������_____________________(����ȷ�𰸱��)��
A���������� B����ȴ�� C�����貹�� D����������
��2��Bװ�õ�������_____________________________________��
��3����װ��C��Ӧ����________(����ȷѡ��ǰ����ĸ)����Ŀ����______________��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ�Ѿ��������������____________________________��
��5��Dװ�þ�֧�Թ���������ˮ����Һ��(�ٶ�������ͬ)���������ŵ�________________��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����_____________�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����_________________________��
ʵ��������������ˮ���������Ҵ��Ʊ�1��2�����������װ������ͼ��ʾ��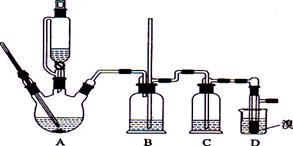
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g �� cm-3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | -l30 | 9 | -1l6 |

�ش��������⣺
��1����ƿD�з�������Ҫ�ķ�Ӧ����ʽ ��
��2����ȫƿB���Է�����,�����Լ��ʵ�����ʱ�Թ�D�Ƿ�����������д����������ʱƿB�е����� ��
��3����װ��C��Ӧ���� (����ĸ) ����Ŀ����_______________��
a��ˮ b��Ũ���� c������������Һ
��4����������������δ��Ӧ��Br2������� ϴ�ӳ�ȥ��������ĸ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��ȡ�������Ҫ���������� ��
��5�������������������������ѣ����� �ķ�����ȥ��
��6����Ӧ������Ӧ����ˮ��ȴװ��D�����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���� ��
��7���жϸ��Ƹ���Ӧ�Ѿ�������������� ��
�Ȼ����dz�����ˮ�����������÷���м���Ʊ���ˮ�Ȼ�����ʵ�����Ʊ�װ�ú�ҵ�Ʊ�����ͼ���£�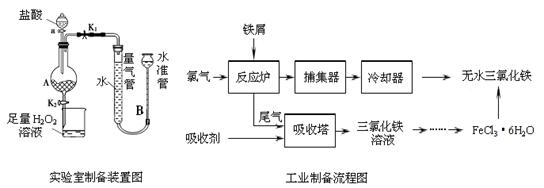
��֪��(1)��ˮFeCl3���۵�Ϊ555 K���е�Ϊ588 K��
(2) ����м�е����ʲ������ᷴӦ
(3)��ͬ�¶�����ˮ���Ȼ�����ˮ�е��ܽ�����£�
| �¶�/�� | 0 | 20 | 80 | 100 |
| �ܽ��(g/100 g H2O) | 74.4 | 91.8 | 525.8 | 535.7 |
ʵ�����Ʊ������������£�
��.���ɼ�K1���رյ��ɼ�K2��������a�������μ����ᡣ
��.�� ʱ���رյ��ɼ�K1�����ɼ�K2����A����Һ��ȫ�����ձ���رջ���a��
��.���ձ�����Һ����һϵ�в�����õ�FeCl3��6H2O���塣
��ش�
��1���ձ���������H2O2��Һ�������� ��
��2��Ϊ�˲ⶨ����м�����������������������С���������������__________��
��3����FeCl3��Һ�Ƶ�FeCl3?6H2O����IJ��������ǣ����� _ �� _ �����ˡ�ϴ�ӡ����
��4����д���������з�Ӧ�����ӷ���ʽ�� ��
��5���������¶ȳ���673 Kʱ��������Է�������Ϊ325�������Ȼ�������ʵķ���ʽΪ ��
��6��FeCl3����������ͨ�����õ������ⶨ����ȡm g��ˮ�Ȼ�����Ʒ������ϡ���ᣬ���Ƴ�100mL��Һ��ȡ��10.00mL�������Թ�����KI��Һ����ַ�Ӧ���뼸�ε�����Һ������c mol?L-1 Na2S2O3��Һ�ζ�������V mL����֪��I2+2S2O32-�T2I-+S4O62-����
�ٵζ��յ�������ǣ� _
����Ʒ���Ȼ������������� _
�����״���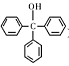 ����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��
����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��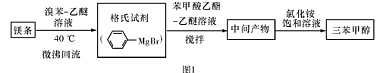
��֪����I�������Լ�����ˮ�⣺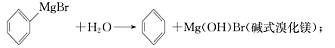
����������ʵ������������£�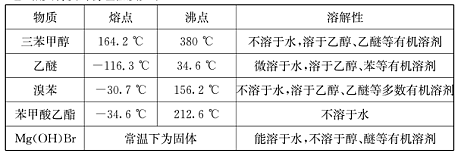
���������״�����Է���������260�����������л���һ�㶼�й̶��۵㡣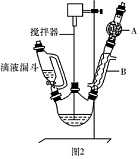
��ش��������⣺
��1��ͼ2�в�������B�����ƣ� ��װ����ˮCaCl2������A�������� ��
��2��ͼ2�еμ�Һ��δ����ͨ��Һ©�����õ�Һ©���������� ����ȡ�����Լ�ʱҪ�����У����Բ��� ���ȷ�ʽ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д���¿հף�
���У��ٲ���Ϊ�� ��ϴ��Һ���ѡ�� ��������ѡ����ѡ��
| A��ˮ | B������ | C���Ҵ� | D���� |
��4�����Ȳⶨ����ȡ2��60 g��Ʒ�����������Һ���������������ƣ��������Ʋ���Ӧ������ַ�Ӧ������ɵ������ڱ�״���µ����Ϊ100.80mL�����Ʒ�������״�����������Ϊ ��
��������ƣ�Na2S2O3��5H2O�����������մ��ֳ�Ϊ�����������ڷ�֯��ҵ��������֯ƷƯ������ȼ���Ⱦë֯�����Ⱦ��������Ⱦ�ϵķ�����ֽ�����ȼ���ҽҩ��ҵ������ϴ�Ӽ�������������ɫ���ȣ���������ˮ���������Ҵ��������ֽ⡣��ҵ�ϳ����������Ʒ�������Ʊ���ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ�����ͼ��
ʵ������������Ϊ��
�ٿ�����Һ©����ʹ�����������£��ʵ����������У�ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ������Ž�����������
����������������ʧ��������Һ��pH�ӽ�7ʱ��ֹͣͨ��SO2 ���塣
�۳������õ���Һ��ת�����������У�ˮԡ����Ũ����ֱ����Һ������־�Ĥ��
����ȴ�ᾧ�����ˡ�ϴ�ӡ�
�ݽ������������У���40��450C���Ҹ���50��60min����������ش��������⣺
��1������b�������� ��
��2���������������PHֵС��7������ʻ��½����������ӷ���ʽ����ԭ�� ��
��3��������в��ܽ���Һ�������ɵ�ԭ���� ����Ĥͨ������Һ������ֵ�ԭ���� ��
��4���������ϴ����������ƾ����Լ��Ľṹʽ�� ��
��5�������йس��˵�˵���У���ȷ���� ��
A��Ϊ�˼���ϴ���Ƿ���ȫ��Ӧ��������ƿ�밲ȫƿ֮����Ƥ�ܣ�������ƿ�Ͽڵ�������ϴ��Һ���Թ��н������ʵ��
B������ǰ�����ܼ�����ֽʪ��ʹ��ֽ��©���ײ�����
C�����˽���ʱӦ�ȹس����ã���������ƿ�ӹ�
D����ϴ�ӳ���ʱ��Ӧ��Сˮ��ͷ��ʹϴ�Ӽ�����ͨ��������
��6��Ϊ�����ƵõIJ�Ʒ�Ĵ��ȣ���ʵ��С���ȡ5.0�˵IJ�Ʒ���Ƴ�250mL�����������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���25mL 0.01mol/L KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+=3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+S4O62-������ɫ��ȥ�Ұ���Ӳ���ɫʱ����ζ��յ㡣ʵ���������±���
| ʵ����� | 1 | 2 | 3 |
| Na2S2O3��Һ�����mL�� | 19.98 | 20.02 | 21.18 |
��ò�Ʒ�Ĵ����� ����ӵ������ζ������п������ʵ����ƫ�͵��� ��
A���ζ���δ��Na2S2O3��Һ��ϴ
B���ζ��յ�ʱ���Ӷ���
C����ƿ������ˮ��ϴ
D���ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢������
E���ζ�ʱ����ƿ�Ͼ���