��Ŀ����
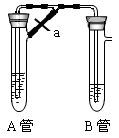
��ҵ������е�Ĺ�������ʾ��ͼ����
���������գ�
(1)����ˮ���������A��B������(A��Դ��ʯ��Ҥ��������B�Ļ�ѧʽ_________��
(2)�ڹ��˺���Һ��Ҫͨ��C��D���壬����ͨ���C������_________(�ѧʽ��,ԭ����_________��
(3)ͨ��C��D���������Ӧ�Ļ�ѧ����ʽ��_________��
(4)�ܹ��˺�����Һ��ͨ����,����ϸСʳ�ο�������������Ʒ________ (��д��ѧʽ����ͨ���������ϸСʳ�ο�����������_________��
(5)д���ݶ��շ�����Ӧ�Ļ�ѧ����ʽ_________����Ʒ�����к���̼�����ƣ����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ���ͯ������(NaHCO3)�� _________ (ע������ʽ�����õ��йط��ŵĺ��壩��
��ÿ��2�֣���(1)��1�֣�����15�֣���1��Na2CO3
��2��NH3����Ϊ��CO2ˮ�е��ܽ��С����NH3�ܽ�ȴ�
��3��NaCl��CO2��NH3��H2O��NaHCO3����NH4Cl
��4��NH4Cl��������Һ�е�Cl����NH4+��Ũ�ȣ�����NH4Cl���ܽ�ȣ�ʹNH4Cl��������
��5��2NaHCO3 Na2CO3��CO2����H2O��
Na2CO3��CO2����H2O�� 
���������������1�������ᴿʱ��Ҫ��ȥ������������Ca2����Mg2����A��Դ��ʯ��Ҥ������A����ʯ�һ���ʯ�ң�������ȥMg2������Ca2��һ����̼��������ȥ����B�Լ���̼���ơ�
��2��������CO2ˮ�е��ܽ��С����NH3�ܽ�ȴ�����Ҫ��ͨ�백����Ȼ����ͨ��CO2���塣
��3������̼�����Ƶ��ܽ�Ƚ�С�������������͵��Ȼ�����Һ��ͨ��CO2��������̼�����ƾ��壬��Ӧ�Ļ�ѧ����ʽ��NaCl��CO2��NH3��H2O��NaHCO3����NH4Cl��
��4��������Һ�к��������Ӻ�NH4+�����Լ���ϸСʳ�ο�����������Һ�е�Cl����NH4+��Ũ�ȣ�����NH4Cl���ܽ�ȣ�ʹNH4Cl���������������������Ʒ���Ȼ�李�
��5��̼�������ȶ��Բ�ڼ��ȵ��������ܷ����ֽ⣬��Ӧ�Ļ�ѧ����ʽΪ2NaHCO3 Na2CO3��CO2����H2O���������ǰ���������Ϊm������ǰ�������Ⱥ������Ϊm�����Ⱥ��������ʧ������Ϊ[m(����ǰ)��m(���Ⱥ�)]����˸��ݷ���ʽ��֪��������̼�����Ƶ�����Ϊ
Na2CO3��CO2����H2O���������ǰ���������Ϊm������ǰ�������Ⱥ������Ϊm�����Ⱥ��������ʧ������Ϊ[m(����ǰ)��m(���Ⱥ�)]����˸��ݷ���ʽ��֪��������̼�����Ƶ�����Ϊ �����Դ����к��е�̼�����Ƶ���������Ϊ
�����Դ����к��е�̼�����Ƶ���������Ϊ ��
��
���㣺�������ʵķ������ᴿ����ѧʵ�����������ʵ�������Ŀ����Լ����ʴ��ȵļ����

 һ����������ϵ�д�
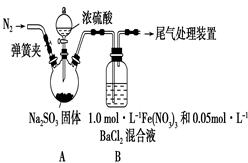
һ����������ϵ�д��������ڿ�����һ�㶼�ױ���������Ħ����[(NH4)2SO4��FeSO4��6H2O]��һ���������Ҫ�ȶ���������ʱ�ֽ��ױ���������ʵ���ҵ��Ʊ�ԭ��Ϊ:FeSO4+(NH4)2SO4+6H2O=(NH4)2SO4��FeSO4��6H2O��
��ͼΪ��ȡĦ���εļ�Ҫ���̣�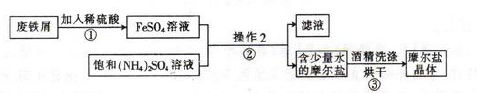
��ش���������:
��1��������з�Ӧ����Ӧ��������ҡ�λ���裬Ŀ���� ��
��2��������еIJ����Ǽ��������� �� ��Ϊʲô���ܼ�������? ��
��3��Ħ������NH4+��Fe2+��SO42-�ļ���:
�ټ�ͬѧ���������ʪ��� ��ֽ��ϡ����� ��Һ���������������ӡ�
����ͬѧ����������е�Fe2+��������KSCN��Һ�� ��Ԥ�ڵ�����ͽ����� ��
��ʵ�ʲ����У���ͬѧ����KSCN��Һʱ��������Һ���dz��ɫ�����������Լ��ķ�������˻��ɡ���ͬѧ������ϸ˼����Ϊ����ͬѧ�ķ����ǿ��еģ�������Ϊ ��
��4����ͬѧ���ⶨĦ������Fe2+�ĺ���������ȡ��4. 0gĦ������Ʒ������ˮ������������ϡ���ᣬ��0.20mol/L��KMnO4��Һ�ζ�������KMnO4��Һ10.00mL
�ٱ�ʵ���ָʾ���� (����ĸ)��
| A����̪ | B��ʯ�� | C������ | D������Ҫ |
�۵ζ��յ��������___ ��
�ܲ�Ʒ��Fe2+�İٷֺ�����___ ��
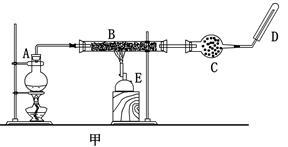
�˽̰桶����1������Fe3+��Fe2+��ת����ʵ��̽����ijУ��ʦΪ�˼���ѧ�����ÿ��ʵ�����ʱ��Ӧ��������˼·�������һ�ݻ�ѧ̽��ѧϰ������-˼·���ʾ���
��1�������±���������д���ÿһ���������̵�˼·
| ���� | ˼· |
| 1.������裺Fe2+���л�ԭ�ԣ����Ա�������Fe3+��Fe3+���������ԣ����Ա���ԭ��Fe2+ | �ٸ���������ԭ��Ӧԭ����һ�� �����л�ԭ�ԣ� ������������ |
| 2.���ʵ��1��ȡ����FeCl2��Һ���μӼ���H2O2��Һ��������Һ�еμӼ���KSCN��Һ���۲���Һ�Ƿ��Ѫ��ɫ | �������ӷ���ʽ��ʾѡ��H2O2��ԭ�� �������ӷ���ʽ��ʾ��Һ��Ѫ��ɫ��ԭ�� |
| 3.���ʵ��2��ȡ����FeCl3������һ�Թܣ�������е�����ˮ�ܽ⣬�μӼ���KSCN��Һ��Ѹ�ټ����������ۣ������Թ������۲���Һ��Ѫ��ɫ�Ƿ���ȥ | ��ѡ�����۵�ԭ���� ���û�ѧ����ʽ��ʾ�� ��ΪʲôҪ����е�ˮ |
| 4.ʵʩʵ�� | ���� |
| ���� | ���� |
��2���̲���ʵ�鷽�����£�ȡ2mLFeCl3��Һ�������������ۣ���ַ�Ӧ���뼸��KSCN��Һ���۲첢��¼ʵ�������ϲ���Һ������һ�Թ��У��ٵ��뼸����ˮ���ַ�����ʲô�仯��ѧ��ͨ��ʵ��֤����ʵ��Ч���ܲû��Ԥ�ڵĺ�ɫ���֣����Ǻܵ���dz��ɫ���Է���ʵ���г����쳣�Ŀ���ԭ���ԸĽ�ʹʵ����������ԡ�
���ܵ�ԭ��
�߸Ľ���ʩ��
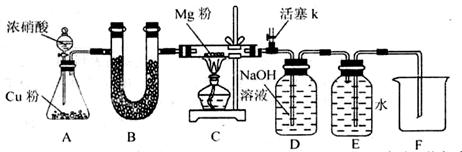
��֪Fe(NO3)2���ȷֽ����Ϊ���������NO2��O2������ɱ������ݲ��ش�������⣺
��1���Թ��������Fe���ϼ۵IJ��룺
����һ��ֻ��+2�� �������ֻ��+3�ۣ��������� ��
��2��Ϊ֤�������ͨ������±�������֤����ѡ�Լ��У� 0.01 mol/L ����KMnO4��Һ��0.1 mol/L H2SO4��Һ��3��H2O2��Һ��0.1 mol/LKSCN��Һ������ˮ��������������
| ʵ����� | ���� | Ŀ�Ļ���� |
| ����1��ȡ���������ȷֽ��Ĺ������Թ��У�����0.1 mol/L H2SO4��Һʹ�����ܽ⣬���ϲ���Һ�ּӵ�A��B��֧�Թ��� | | �ܽ������� |
| ����2�� | | ��Һ�к���Fe3+ |
| ����3�� | | ��Һ�в��� Fe2+ |
����֤���������ȷ����д��Fe(NO3)2�ȷֽ�Ļ�ѧ����ʽ ��
��3��ijͬѧ�������ǵ�ľ������÷ֽⷴӦ�����������У�ľ����ȼ���ɴ����ó���NO2��֧��ȼ�յĽ��ۡ��������жϸý����Ƿ���ȷ����˵�����ɡ�
���й���Ũ�����Ũ�������������ȷ����( )
| A�������¶����������������� |
| B�������¶�����ͭ�Ͽ췴Ӧ |
| C��¶���ڿ����У���������Һ���������� |
| D��¶���ڿ����У���������Һ��Ũ�Ⱦ�����ˮ������ |