��Ŀ����
�������ڿ�����һ�㶼�ױ���������Ħ����[(NH4)2SO4��FeSO4��6H2O]��һ���������Ҫ�ȶ���������ʱ�ֽ��ױ���������ʵ���ҵ��Ʊ�ԭ��Ϊ:FeSO4+(NH4)2SO4+6H2O=(NH4)2SO4��FeSO4��6H2O��
��ͼΪ��ȡĦ���εļ�Ҫ���̣�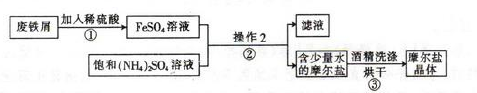
��ش���������:
��1��������з�Ӧ����Ӧ��������ҡ�λ���裬Ŀ���� ��
��2��������еIJ����Ǽ��������� �� ��Ϊʲô���ܼ�������? ��
��3��Ħ������NH4+��Fe2+��SO42-�ļ���:
�ټ�ͬѧ���������ʪ��� ��ֽ��ϡ����� ��Һ���������������ӡ�
����ͬѧ����������е�Fe2+��������KSCN��Һ�� ��Ԥ�ڵ�����ͽ����� ��
��ʵ�ʲ����У���ͬѧ����KSCN��Һʱ��������Һ���dz��ɫ�����������Լ��ķ�������˻��ɡ���ͬѧ������ϸ˼����Ϊ����ͬѧ�ķ����ǿ��еģ�������Ϊ ��
��4����ͬѧ���ⶨĦ������Fe2+�ĺ���������ȡ��4. 0gĦ������Ʒ������ˮ������������ϡ���ᣬ��0.20mol/L��KMnO4��Һ�ζ�������KMnO4��Һ10.00mL
�ٱ�ʵ���ָʾ���� (����ĸ)��
| A����̪ | B��ʯ�� | C������ | D������Ҫ |
�۵ζ��յ��������___ ��
�ܲ�Ʒ��Fe2+�İٷֺ�����___ ��
��1����ֹFe2����������1�֣�
��2����ȴ�ᾧ�����ˣ�Ħ���������ֽ��ױ���������1�֣�
��3���ٺ�ɫʯ�������������1�֣�
����ˮ����˫��ˮ����1�֣�������KSCN��Һ�����������ټ�����ˮ����Һ��죬��֤����Һ�к���Fe2����2�֣�
�ۼ���������ˮ����Һ��ɫ���������֤����Һ�к���Fe2����1�֣�
��4����D��1�֣�
����ʽ��1�֣�
�۵������һ��KMnO4��Һҡ�Ⱥ���ƿ�е���Һ��dz��ɫ��Ϊdz��ɫ����30 s�ڲ���ɫ��2�֣�
��14%��2�֣�
���������������1����������ҡ�λ���裬��ֹFe2����������
��2������Ҫ��ȴ�ᾧ�����ˣ�Ħ���������ֽ��ױ�������
��3����������ٺ�ɫʯ���������������ˮ����˫��ˮ��������KSCN��Һ�����������ټ�����ˮ����Һ��죬��֤����Һ�к���Fe2�����ۼ���������ˮ����Һ��ɫ���������֤����Һ�к���Fe2����
��4����KMnO4��Һ������ɫ������Ҫָʾ����
�����ԡ��������Լ�Ӧ��ѡ����ʽ�ζ��ܡ�
�۵������һ��KMnO4��Һҡ�Ⱥ���ƿ�е���Һ��dz��ɫ��Ϊdz��ɫ����30 s�ڲ���ɫ��
�ܸ��ݵ����غ�KMnO4~5 Fe2+������n(Fe2��)=5��10��10-3 L��0.20mol/L=10-2 mol,Fe2+�İٷֺ���Ϊ10-2 mol��56g/mol��4.0g��100%=14%��
���㣺����Ԫ�ؼ��仯�������ʡ���ѧʵ��֪ʶ����ѧ��Ӧ�ļ��㡣

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�I������ռ������Ҫ�Ļ���ԭ�ϡ�
��1��������ͼ��ʾװ�ÿɼ��֤��������̼���ռ���Һ�����˷�Ӧ����A��B���ӣ���ֹˮ�У�����ͷ�ι��е�Һ�強����ƿ����ʱ��ʵ��������___________________��
�������������䣬��A��C���ӣ��ɹ۲쵽��������__________________________��
��2����NaOH��Һ��ͨ��һ����CO2���ᾧ��õ���ɫ���壬�ð�ɫ�������ɿ����ǣ�
A��NaOH��Na2CO3��B����������������C������������������D����������������
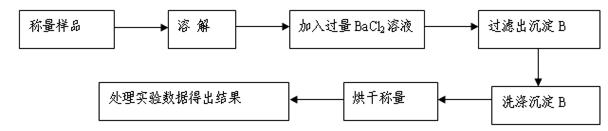
��3�����ʵ��ȷ����2���а�ɫ�����д���A���е������ӣ�
| ʵ����� | ʵ������ | ���� |
| ��ȡ������ɫ�������Թ��У�������ˮ�ܽ⣬�ټ�����BaCl2��Һ | | |
| �� | | |
II����ѧ��ȤС���ijƷ�������е�Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ƣ�����������ɣ������������ɷ���������ʱ�����������
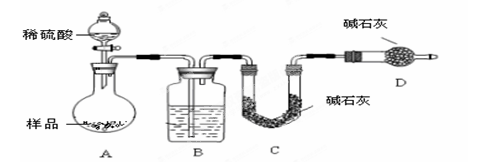
������Ʒ��̼��ƵĶ����ⶨ��������ͼ��ʾװ��(ͼ�мг�������ȥ)����ʵ�飬��ַ�Ӧ
�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

����ʵ����̻ش��������⣺
��1��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���У� ��
��2��C�з�Ӧ����BaCO3�����ӷ���ʽ�� ��
��3�����и����ʩ�У�������߲ⶨȷ�ȵ��ǣ� ��
A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B���μ�����˹���
C����A��B֮������ʢ��Ũ�����ϴ��װ��
D����B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��4��ʵ����ȷ��ȡ8.00 g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94 g.����Ʒ��̼��Ƶ���������Ϊ________��
��5��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����_________________________________��
��6��װ����U�ι�D�еļ�ʯ�ҵ�������_____________________________��
ijУ��ѧ��ȤС��Ϊ̽������Ũ����ķ�Ӧ,�����ͼ1��ͼ2��ʾװ�ý���ʵ�顣 
��1����˵����SO2���������ʵ�������� ��
��2��ͼ2�е�����e����Ҫ����Ϊ ��
��3������װ����ͼ2�е�NaOH��Һ������SO2β������ֹ��Ⱦ���罫�����Ϊ����KMnO4��Һ��ͬ�����ԴﵽĿ�ģ���д������KMnO4��Һ��SO2��Ӧ�Ļ�ѧ����ʽ��
��
��4���Ա�����ʵ��װ�ã����ѷ���ͼ2װ�ó����ܸ��õ������ж�����SO2��ֹ����Ⱦ�����⣬����һ���dz����Ե��ŵ㣬����Ϊ�� ��
��5����Ӧһ��ʱ���ֹͣ��Ӧ������ȴ���ý�ͷ�ι���ȡA�Թ��е���Һ���뵽����ˮ����Ϊ�����������������������ӵijɷ����������ֿ��ܣ�
��ֻ����Fe3+����ֻ����Fe2+�� ����Fe3+����Fe2+��
Ϊȷ����Һ�ijɷ֣�ѡ�������Լ���
| A��ϡHCl��Һ | B��ϡ���� | C��KSCN��Һ | D������KMnO4��Һ |
�����������ص�ʵ��̽����
| ʵ�鲽�� | ʵ�������� |
| 1��ȡһ֧�ྻ���Թܣ��μ�1-2mL��������Һ�������Թ��еμӼ���KSCN��Һ | ��1�� ����˵��������� ��2�� ����˵����Һ�д���Fe3+������������ |
| 2�� | �� |
�������������������Ӧʱ���������Ԫ�صĻ��ϼ۲��ᷢ���仯���ǣ�������
| A��ϡ���� | B��ϡ���� | C��Ũ���� | D��Ũ���� |






 2Al2O3+9SO2���÷�Ӧ����������_______��������l molAl2O3����ת�Ƶĵ�����Ϊ__________________��
2Al2O3+9SO2���÷�Ӧ����������_______��������l molAl2O3����ת�Ƶĵ�����Ϊ__________________��