��Ŀ����
ij����С�����Mg��CO2�ķ�Ӧԭ����̽��Mg��NO2�ķ�Ӧ����������С��ͨ��ʵ��ȷ��Mg����NO2��ȼ�գ����Թ������������ּ��裺
I��ͬ�����ΪMgO��
II������Ϊ______________��
III��������______________��
��ش��������⣺
������Ϣ��2NO2 + 2NaOH = NaNO3 + NaNO2 + H2O
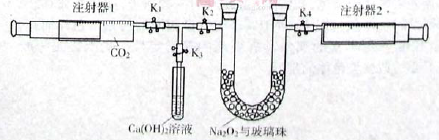
��1����ͼ���Ӻ�������װҩƷǰ��μ���װ�õ�������_______��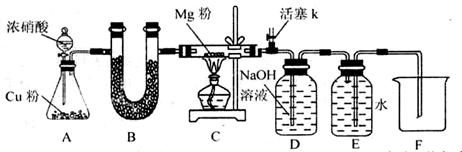
��2��װ��B��ʢװ�ĸ���������ǣ�����ţ�_______��
��Ũ���� ����ˮCaCl2 �ۼ�ʯ�� ������������
��3����ʼ����k����A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ�Ķ���______________��
��4��E���ռ��������������������ܶ���14����������_______��
��5��ʵ��õ�����������������ʵ��ǰMg��������1.5���������_______������C�з����Ļ�ѧ��Ӧ����ʽΪ______________
��6����ʵ���д�������ȱ�ݣ��Ľ���ʩ��___________
�������ΪMg3N2
III���������ΪMgO��Mg3N2
��1���رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á�
��2���ڢ�
��3���ž�װ���п�������ֹ��������ʵ�顣
��4��N2
��5������III 4Mg+2NO2 4MgO+N2 3Mg+N2
4MgO+N2 3Mg+N2 Mg3N2
Mg3N2
��6������K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
�������������Mg��NO2��Ӧ������ԭ���غ㣬����������MgO��Mg3N2��MgO��Mg3N2
��1������װ��������ͨ���ü��ȷ����������������رգ�������ĩ������ˮ�У������巢��װ�ã����Ƿ�������ݣ�ֹͣ���Ⱥ��������Ƿ���һ��ˮ����
��2��NO2����Ũ���ᡢNaOH��Ӧ����˲�����Ũ���ᡢ��ʯ�Ҹ��
��3��Ϊ��ֹMg������е�N2��O2��Ӧ��ʵ��������ţ����ž�װ���п�����
��4���ռ������������������ܶ���14�����������Է���������2��14=28������ΪN2��
��5��������ֻ��MgO������������Ƿ�Ӧǰ��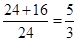 ��1.7����������ֻ��Mg3N2�������������Ƿ�Ӧǰ��
��1.7����������ֻ��Mg3N2�������������Ƿ�Ӧǰ��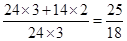 ��1.4����ʵ��õ��Ĺ��������Ƿ�Ӧǰ��1.5������õ�����MgO��Mg3N2�Ļ����ʼ���III������
��1.4����ʵ��õ��Ĺ��������Ƿ�Ӧǰ��1.5������õ�����MgO��Mg3N2�Ļ����ʼ���III������
����Mg��CO2�ķ�Ӧ����֪Mg��NO2��Ӧ����MgO��N2������N2������Mg��Ӧ�õ�Mg3N2��
��6���Ƚ����Ե�ȱ���ǣ��ž�װ�ÿ���ʱ��������NO2�ڻ���k��ֱ����������У�����Ⱦ����������轫����k���ĵ���ͨ��NaOH��Һ�С�
���㣺���黯ѧʵ��ԭ�������������Լ�飬�Լ�ѡ��ʵ��װ�õ����ã�ʵ�����ݴ�����ʵ��װ�øĽ��ȡ�

CuSO4��һ����Ҫ�Ļ���ԭ�ϣ��й��Ʊ�;������������ͼ��ʾ������˵���������( )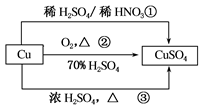
| A��;�������û�����H2SO4��HNO3��������ʵ���֮��Ϊ3��2 |
| B����;���١�����ȣ�;���ڸ��õ���������ɫ��ѧ˼�� |
| C�����ɵ���������ͭ������;���вμӷ�Ӧ����������ʵ�����=��<�� |
| D������;�����Ʊ�16g����ͭ������ԭ����������ʵ���Ϊ0.1mol |
�Ŵ������ϵ�����������û�з�������Ȼ���ڵļ��ء�20����80�����ѧ�ҽ��г��������о�ʱ��żȻ������ɷ�Ϊ��ɫ�Ĺ���ͭ��(��ѧʽ��BaCuSi2Ox��CuΪ��2��)�������йء����ϡ���˵���в���ȷ����
| A�����ε���ʽ��ʾ�� BaSiO3��CuSiO3 | B������������ʽ��ʾ��BaO��CuO��2SiO2 |
| C��������ǿ�ᡢǿ�� | D�������ȶ���������ɫ |
�屻��Ϊ������Ԫ�ء������Ԫ�ط�����
| A��B | B��Be | C��Br | D��Bi |
 �ⶨʣ���������
�ⶨʣ��������� �ⶨ��������
�ⶨ�������� �ⶨ���ɶ�����̼������
�ⶨ���ɶ�����̼������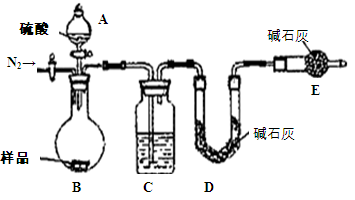

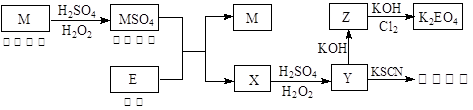
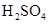 ��
��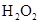 ���Һ�����ӷ���ʽ�� ��
���Һ�����ӷ���ʽ�� ��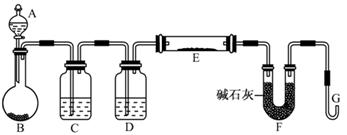

 2Al2O3+9SO2���÷�Ӧ����������_______��������l molAl2O3����ת�Ƶĵ�����Ϊ__________________��
2Al2O3+9SO2���÷�Ӧ����������_______��������l molAl2O3����ת�Ƶĵ�����Ϊ__________________��