��Ŀ����
����Ŀ����֪ͼһ��ʾ���ǿ��淴ӦCO(g)��H2(g) ![]() C(s)��H2O(g) ��H��0�Ļ�ѧ��Ӧ����(v)��ʱ��(t)�Ĺ�ϵ��ͼ����ʾ���ǿ��淴Ӧ2NO2(g)
C(s)��H2O(g) ��H��0�Ļ�ѧ��Ӧ����(v)��ʱ��(t)�Ĺ�ϵ��ͼ����ʾ���ǿ��淴Ӧ2NO2(g) ![]() N2O4(g) ��H��0��Ũ��(c)��ʱ��t�ı仯���������˵������ȷ����
N2O4(g) ��H��0��Ũ��(c)��ʱ��t�ı仯���������˵������ȷ����
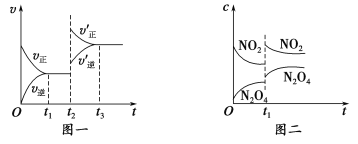
A��ͼһt2ʱ�ı�������������������¶Ȼ�ʹ���˴���
B����ͼһt2ʱ�ı������������ѹǿ����Ӧ�Ħ�H����
C��ͼ��t1ʱ�ı�������������������¶Ȼ�������ѹǿ
D����ͼ��t1ʱ�ı������������ѹǿ�����������ƽ����Է�������������
���𰸡�D
��������
��������� A��CO(g)��H2(g) ![]() C(s)��H2O(g) ��H��0�������¶�ƽ��������У����������ı�ƽ��״̬��A����B��CO(g)��H2(g)
C(s)��H2O(g) ��H��0�������¶�ƽ��������У����������ı�ƽ��״̬��A����B��CO(g)��H2(g)![]() C(s)��H2O(g)����Ӧ�������С�Ŀ��淴Ӧ��������ѹǿƽ��������Ӧ�������������Ӧ����ƽ���ƶ�����û�й�ϵ��B����C��2NO2(g)
C(s)��H2O(g)����Ӧ�������С�Ŀ��淴Ӧ��������ѹǿƽ��������Ӧ�������������Ӧ����ƽ���ƶ�����û�й�ϵ��B����C��2NO2(g) ![]() N2O4(g) ��H��0���������¶ȣ�ƽ�������ƶ���t1ʱ�̺�c��NO2��������c��N2O4����С��C����D����
N2O4(g) ��H��0���������¶ȣ�ƽ�������ƶ���t1ʱ�̺�c��NO2��������c��N2O4����С��C����D����![]() ���Է�Ӧ2NO2(g)
���Է�Ӧ2NO2(g)![]() N2O4(g)������ѹǿ��ƽ�������ƶ���
N2O4(g)������ѹǿ��ƽ�������ƶ���![]() ��С��
��С��![]() Ӧ������D��ȷ����ѡD��
Ӧ������D��ȷ����ѡD��

����Ŀ��10��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
�¶ȣ��棩 | 10 | 20 | 30 | ������к���ȴ��50�� |
pH | 8.3 | 8.4 | 8.5 | 8.8 |
�����������ݣ���ͬѧ��Ϊ������Һ��pH���ߵ�ԭ����HCO3����ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ____________________________����ͬѧ��Ϊ����ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�Na2CO3��ˮ��̶�_______����������������С������NaHCO3����ͬѧ��Ϊ�ס��ҵ��ж϶�����֡�����Ϊ��
��1��ֻҪ�ڼ�����е���Һ�м����������Լ�X����������������____�������������������ж���ȷ���Լ�X��_____________������ţ���
A��Ba(OH)2��Һ B��BaCl2��Һ C��NaOH��Һ D�������ʯ��ˮ
��2�������Ⱥ����Һ��ȴ��10��������Һ��pH_____(��������������������������������8.3����_____���������������������ж���ȷ��
��3���������ϣ�����NaHCO3�ķֽ��¶�Ϊ150����������_____���������������������ж��Ǵ���ģ�������_____________________________________________________��