��Ŀ����
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������(�������в�����Ԫ�غ���Ԫ�أ������ʲ������ᷴӦ)��ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
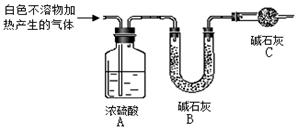
��.����ʯ�к������IJⶨ������ʵ����̲��������벹��������
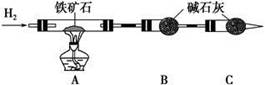
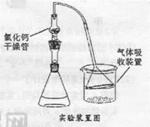
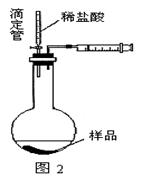
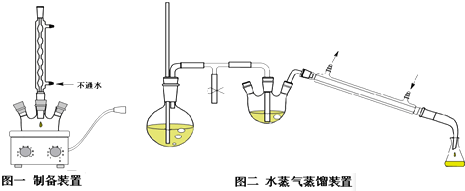
(1)����ͼ��װ��������______________________________________________��
(2)��8.0 g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ(�г�������ʡ��)��
(3)����˵����ܿڴ����ϵػ���ͨ��H2��____________________________��
��ȼA���ƾ��ƣ�
(4)��ַ�Ӧ�����ƾ��ƣ�________________________________________��
(5)��÷�Ӧ��װ��B����2.25 g��������ʯ�����İٷֺ���Ϊ________��
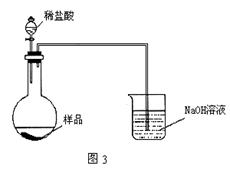
��.����ʯ�к������IJⶨ���������¡�
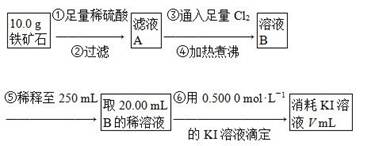
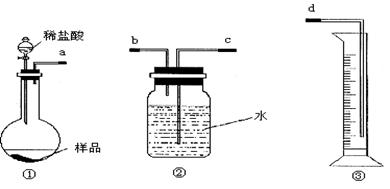
(1)���������������___________________________________________��
(2)��������õ��IJ����������ձ�����ͷ�ιܡ�250 mL����ƿ��________��
(3)�����йز���IJ�����˵����ȷ����________��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ������п����õ�����Һ��ָʾ��
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
f���ζ������ζ��ܼ��첿�������ݣ���ⶨ���ƫ��
(4)���ζ�����������0.500 0 mol��L��1 KI��Һ20.00 mL��������ʯ�����İٷֺ���Ϊ________��
��.�ɢ���������������ʯ������������Ļ�ѧʽΪ________��
��.����ʯ�к������IJⶨ������ʵ����̲��������벹��������

(1)����ͼ��װ��������______________________________________________��
(2)��8.0 g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ(�г�������ʡ��)��
(3)����˵����ܿڴ����ϵػ���ͨ��H2��____________________________��
��ȼA���ƾ��ƣ�
(4)��ַ�Ӧ�����ƾ��ƣ�________________________________________��
(5)��÷�Ӧ��װ��B����2.25 g��������ʯ�����İٷֺ���Ϊ________��
��.����ʯ�к������IJⶨ���������¡�

(1)���������������___________________________________________��
(2)��������õ��IJ����������ձ�����ͷ�ιܡ�250 mL����ƿ��________��
(3)�����йز���IJ�����˵����ȷ����________��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ������п����õ�����Һ��ָʾ��
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
f���ζ������ζ��ܼ��첿�������ݣ���ⶨ���ƫ��
(4)���ζ�����������0.500 0 mol��L��1 KI��Һ20.00 mL��������ʯ�����İٷֺ���Ϊ________��
��.�ɢ���������������ʯ������������Ļ�ѧʽΪ________��
��.(1)���װ�õ������ԡ�(3)��װ��C���ڴ������鴿��(4)�ٳ���ͨ��������Ӳ�ʲ�������ȫ��ȴ��(5)25.0%
��.(1)������Һ���ܽ�Ĺ���Cl2��(2)��������(3)be��(4)70.0%
��.Fe4O5
��.(1)������Һ���ܽ�Ĺ���Cl2��(2)��������(3)be��(4)70.0%
��.Fe4O5
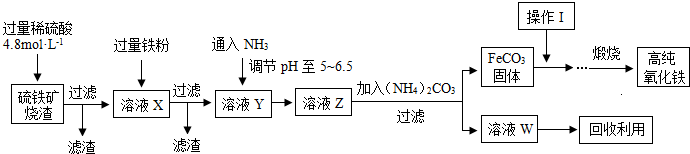
���⿼������ʯ������������Ļ�ѧʽ��̽����
��.ʵ�鿪ʼǰһ��Ҫ�ȼ��װ�õ������ԣ���װ��ҩƷ���ڵ�ȼA���ƾ���֮ǰһ��Ҫ�ȼ��������Ĵ��ȣ������ƾ��ƺ�һ��Ҫ��ͨ������һ��ʱ�䣬ֱ��Ӳ�ʲ�������ȴ�����£��Է������±�������װ��B���ӵ�������Ϊ����ˮ������������m(O)��2.25 g��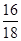 ��2.0 g����Ԫ�ص���������Ϊ
��2.0 g����Ԫ�ص���������Ϊ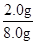 ��100%��25.0%����.(1)��Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ��������(2)����һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢������(3)��ˮ�Ļ�ɫ�����ԣ�a���ζ�ʱ������Ӧ��2Fe3����2I��===2Fe2����I2���ζ�ʱ���������ۣ�����Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ������ζ��ܼ��첿�������ݣ�������KI��Һ������ƫ�ͣ��ⶨ���ƫ�ͣ�f����
��100%��25.0%����.(1)��Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ��������(2)����һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢������(3)��ˮ�Ļ�ɫ�����ԣ�a���ζ�ʱ������Ӧ��2Fe3����2I��===2Fe2����I2���ζ�ʱ���������ۣ�����Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ������ζ��ܼ��첿�������ݣ�������KI��Һ������ƫ�ͣ��ⶨ���ƫ�ͣ�f����
(4)����2Fe3����2I��===2Fe2����I2��֪10.0 g����ʯ�У�n(Fe)��0.500 0 mol��L��1��0.02 L��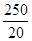 ��0.125 mol��m(Fe)��7.0 g������Ԫ�ص���������Ϊ70.0%����.�����������У�n(Fe)��n(O)��
��0.125 mol��m(Fe)��7.0 g������Ԫ�ص���������Ϊ70.0%����.�����������У�n(Fe)��n(O)��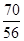 ��
��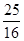 ��4��5�������仯ѧʽΪFe4O5��
��4��5�������仯ѧʽΪFe4O5��
��.ʵ�鿪ʼǰһ��Ҫ�ȼ��װ�õ������ԣ���װ��ҩƷ���ڵ�ȼA���ƾ���֮ǰһ��Ҫ�ȼ��������Ĵ��ȣ������ƾ��ƺ�һ��Ҫ��ͨ������һ��ʱ�䣬ֱ��Ӳ�ʲ�������ȴ�����£��Է������±�������װ��B���ӵ�������Ϊ����ˮ������������m(O)��2.25 g��
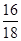 ��2.0 g����Ԫ�ص���������Ϊ
��2.0 g����Ԫ�ص���������Ϊ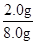 ��100%��25.0%����.(1)��Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ��������(2)����һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢������(3)��ˮ�Ļ�ɫ�����ԣ�a���ζ�ʱ������Ӧ��2Fe3����2I��===2Fe2����I2���ζ�ʱ���������ۣ�����Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ������ζ��ܼ��첿�������ݣ�������KI��Һ������ƫ�ͣ��ⶨ���ƫ�ͣ�f����
��100%��25.0%����.(1)��Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ��������(2)����һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢������(3)��ˮ�Ļ�ɫ�����ԣ�a���ζ�ʱ������Ӧ��2Fe3����2I��===2Fe2����I2���ζ�ʱ���������ۣ�����Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ������ζ��ܼ��첿�������ݣ�������KI��Һ������ƫ�ͣ��ⶨ���ƫ�ͣ�f����(4)����2Fe3����2I��===2Fe2����I2��֪10.0 g����ʯ�У�n(Fe)��0.500 0 mol��L��1��0.02 L��
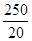 ��0.125 mol��m(Fe)��7.0 g������Ԫ�ص���������Ϊ70.0%����.�����������У�n(Fe)��n(O)��
��0.125 mol��m(Fe)��7.0 g������Ԫ�ص���������Ϊ70.0%����.�����������У�n(Fe)��n(O)��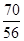 ��
��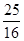 ��4��5�������仯ѧʽΪFe4O5��
��4��5�������仯ѧʽΪFe4O5��
��ϰ��ϵ�д�
 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
�����Ŀ
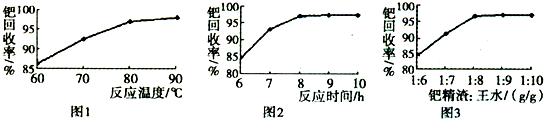
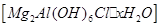 ��һ���������ӽ������ϣ����ڸ�������ȫ�ֽ�Ϊ
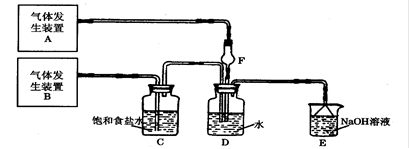
��һ���������ӽ������ϣ����ڸ�������ȫ�ֽ�Ϊ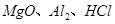 ��ˮ������������27ͼװ�ý�������ȷ���仯ѧʽ���̶�װ����ȥ����
��ˮ������������27ͼװ�ý�������ȷ���仯ѧʽ���̶�װ����ȥ����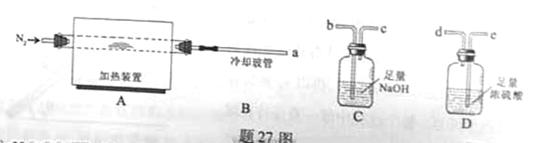
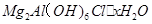 �ȷֽ�Ļ�ѧ����ʽΪ ��
�ȷֽ�Ļ�ѧ����ʽΪ ��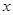 ,��װ�õ�����˳��Ϊ �������������ýӿ���ĸ��ʾ��������C�������� ��װ�����Ӻ�����Ҫ���еIJ��������� ��
,��װ�õ�����˳��Ϊ �������������ýӿ���ĸ��ʾ��������C�������� ��װ�����Ӻ�����Ҫ���еIJ��������� ��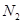 �ž�װ���еĿ�������ȡC��D�ij�ʼ�������ٳ���ͨ��
�ž�װ���еĿ�������ȡC��D�ij�ʼ�������ٳ���ͨ��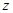 �����ⶨD�������⣬���ٻ���ⶨ .
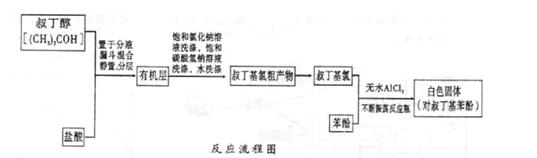
�����ⶨD�������⣬���ٻ���ⶨ .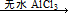 ArR+HX ��H<0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�����е�90.70C)�����ᷴӦ�Ƶ��嶡���ȣ��е�500C)��������Fridel��Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�990C)����Ӧ���̼�ʵ��װ������ͼ��ʾ��
ArR+HX ��H<0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�����е�90.70C)�����ᷴӦ�Ƶ��嶡���ȣ��е�500C)��������Fridel��Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�990C)����Ӧ���̼�ʵ��װ������ͼ��ʾ��