��Ŀ����
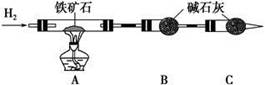
��12�֣�ijѧ��ʵ����ȤС������ͼ1װ������ɡ�NaHCO3��NaCl�������NaHCO3�����IJⶨ����ʵ�顣
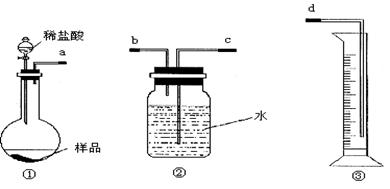
ͼ1
��1���������ӿ�����˳����_________________���ýӿ���ĸ��д����
��2����װ�����Ӻö�δװҩƷǰ�����������ԵIJ�����_________________________��
��3����ͬѧ��Ϊ�����������ϴ�������¸Ľ���ʩ������Ϊ���е���______��������ţ�
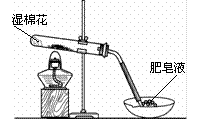
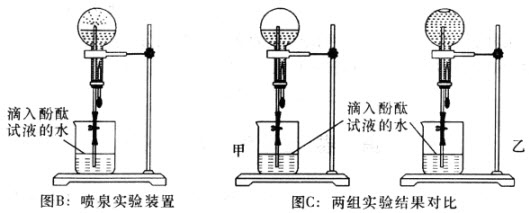
��4����ͬѧ��Ϊ��װ�âڢ�֮��ĵ����ڻ����ˮ��ʹ�ⶨ��������Ӷ�����ͼ2װ�á��ٶ��ζ�����ʼ����ΪV1mL�����˶���ΪV2mL����ע�����ⶨ�ų�������ΪV3mL����״���£����������Ʒ����Ϊm g����ԭ�������NaHCO3�����������ı���ʽΪ_______��
���ú�V1��V2��V3��m��ʽ�ӱ�ʾ����
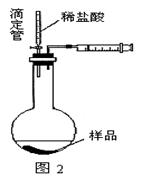
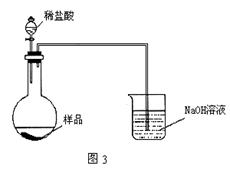
��5����ͬѧ����ͼ3װ�ã�ͨ���ⶨ�ձ�������������Ϊ�ҡ�����ͬѧ�ķ����У�˭�ķ���������__________��������:_____________��

ͼ1
��1���������ӿ�����˳����_________________���ýӿ���ĸ��д����
��2����װ�����Ӻö�δװҩƷǰ�����������ԵIJ�����_________________________��
��3����ͬѧ��Ϊ�����������ϴ�������¸Ľ���ʩ������Ϊ���е���______��������ţ�
| A���ڵ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���� |
| B����װ�â���ϡ���ỻ��ϡ���ᣬװ�â���ˮ���ɱ���NaHCO3��Һ |
| C����װ�â���ˮ���ɱ���Na2CO3��Һ |
| D���μ�����˹��� |
���ú�V1��V2��V3��m��ʽ�ӱ�ʾ����


��5����ͬѧ����ͼ3װ�ã�ͨ���ⶨ�ձ�������������Ϊ�ҡ�����ͬѧ�ķ����У�˭�ķ���������__________��������:_____________��
��1��a��b��c��d��2�֣�
��2����1���ڢ��м�ˮ��û�����ܣ��رշ�Һ©��������������סԲ����ƿ����ƾ����ȣ��������г�������Һ����������˵�������Ժã���2���ڢ��м�ˮ��û���ܣ��رշ�Һ©���������þƾ����ȣ������е��ܿڲ������ȵ����ݣ�ֹͣ���Ⱥ���ĩ���γ�һ��ˮ���ұ���һ��ʱ�䲻�½�����˵�������Ժá���2�֣�
��3��BD��2�֣�
��4��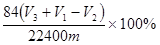 ��2�֣�
��2�֣�
��5����ͬѧ��1�֣���ͬѧ�ķ����У����ɵ�CO2û��ȫ��NaOH��Һ���ա���2�֣�
��2����1���ڢ��м�ˮ��û�����ܣ��رշ�Һ©��������������סԲ����ƿ����ƾ����ȣ��������г�������Һ����������˵�������Ժã���2���ڢ��м�ˮ��û���ܣ��رշ�Һ©���������þƾ����ȣ������е��ܿڲ������ȵ����ݣ�ֹͣ���Ⱥ���ĩ���γ�һ��ˮ���ұ���һ��ʱ�䲻�½�����˵�������Ժá���2�֣�
��3��BD��2�֣�
��4��
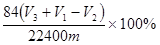 ��2�֣�
��2�֣���5����ͬѧ��1�֣���ͬѧ�ķ����У����ɵ�CO2û��ȫ��NaOH��Һ���ա���2�֣�
��1������ʵ��װ��ͼ��֪��������ͨ����ˮ������ˮ������������ó�����������ʵ����ȷ��˳����a��b��c��d��
��2������װ�õ��ص��֪�����鷽�����ڢ��м�ˮ��û�����ܣ��رշ�Һ©��������������סԲ����ƿ����ƾ����ȣ��������г�������Һ����������˵�������Ժã����ڢ��м�ˮ��û���ܣ��رշ�Һ©���������þƾ����ȣ������е��ܿڲ������ȵ����ݣ�ֹͣ���Ⱥ���ĩ���γ�һ��ˮ���ұ���һ��ʱ�䲻�½�����˵�������Ժá�
��3�����ڷ�Ӧ��װ���л��������CO2���壬���Է�Ӧǰû�б�Ҫ�ž�װ���ڵ�CO2���塣����CO2�ܺ�̼������Һ��Ӧ������CҲ����ȷ�������ȷ�Ĵ���BD��
��4����������ı仯��֪�����ɵ�CO2��V3����V2��V1����V3��V1��V2�������ʵ����ǣ�V3��V1��V2��/22400mol������̼�����Ƶ�������84��V3��V1��V2��/22400g���������������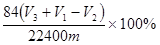 ��
��
��3����װ�â۵Ľṹ��֪����Ӧ�в�����CO2��������ȫ������������Һ���գ��ⶨ���ƫ�ͣ����Բ���װ�âںá�
��2������װ�õ��ص��֪�����鷽�����ڢ��м�ˮ��û�����ܣ��رշ�Һ©��������������סԲ����ƿ����ƾ����ȣ��������г�������Һ����������˵�������Ժã����ڢ��м�ˮ��û���ܣ��رշ�Һ©���������þƾ����ȣ������е��ܿڲ������ȵ����ݣ�ֹͣ���Ⱥ���ĩ���γ�һ��ˮ���ұ���һ��ʱ�䲻�½�����˵�������Ժá�
��3�����ڷ�Ӧ��װ���л��������CO2���壬���Է�Ӧǰû�б�Ҫ�ž�װ���ڵ�CO2���塣����CO2�ܺ�̼������Һ��Ӧ������CҲ����ȷ�������ȷ�Ĵ���BD��
��4����������ı仯��֪�����ɵ�CO2��V3����V2��V1����V3��V1��V2�������ʵ����ǣ�V3��V1��V2��/22400mol������̼�����Ƶ�������84��V3��V1��V2��/22400g���������������
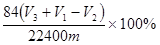 ��
����3����װ�â۵Ľṹ��֪����Ӧ�в�����CO2��������ȫ������������Һ���գ��ⶨ���ƫ�ͣ����Բ���װ�âںá�

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
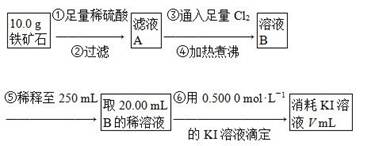
 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�