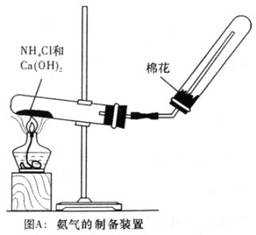
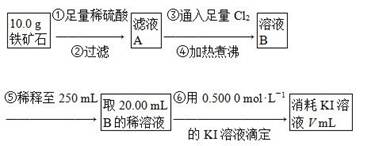
��Ŀ����
Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ���������ͼ��ʾ��ʵ��װ�á�����գ�
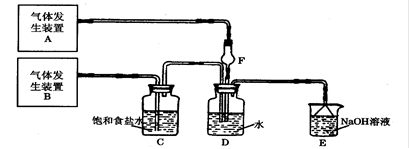
��1��Dװ������Ҫ��Ӧ�����ӷ���ʽΪ ��F������������ ��
��2��Ϊ��֤ͨ��Dװ���е�������Cl2����SO2��������ȤС���ͬѧ���������Լ���
a �Ȼ���ϡ��Һ b �Ȼ�������Һ c ���軯����Һ
d ������Һ e Ʒ����Һ f ���Ը��������Һ
����Cl2������ȡ����D����Һ�μ���ʢ�� ��ѡ��һ�����ţ��Լ����Թ��ڣ��ټ��� ��ѡ��һ�����ţ��Լ��������������ǣ� ��
����ѡ�������Լ��е�һ���ٽ�ϱ�Ҫ�IJ����Ϳ�����֤ͨ��Dװ���е�������Cl2��������SO2���������Լ��� ��ѡ��һ�����ţ�����Ҫ�IJ����ͽ����� ��

��1��Dװ������Ҫ��Ӧ�����ӷ���ʽΪ ��F������������ ��
��2��Ϊ��֤ͨ��Dװ���е�������Cl2����SO2��������ȤС���ͬѧ���������Լ���
a �Ȼ���ϡ��Һ b �Ȼ�������Һ c ���軯����Һ
d ������Һ e Ʒ����Һ f ���Ը��������Һ
����Cl2������ȡ����D����Һ�μ���ʢ�� ��ѡ��һ�����ţ��Լ����Թ��ڣ��ټ��� ��ѡ��һ�����ţ��Լ��������������ǣ� ��
����ѡ�������Լ��е�һ���ٽ�ϱ�Ҫ�IJ����Ϳ�����֤ͨ��Dװ���е�������Cl2��������SO2���������Լ��� ��ѡ��һ�����ţ�����Ҫ�IJ����ͽ����� ��
����12�֣���1��Cl2+SO2+2H2O��4H++2Cl�D+SO42�� ��2�֣��� ������(2��)��
��2���� b (1��)�� c����d��(1��)�� ��Һ�ʺ�ɫ������ɫ��(2��)��
�� e��2�֣���Ʒ����ɫ�����Ȳ���ԭ��˵������������Ʒ����ɫ�������ָ�ԭ��˵�����������������2�֣�
��2���� b (1��)�� c����d��(1��)�� ��Һ�ʺ�ɫ������ɫ��(2��)��
�� e��2�֣���Ʒ����ɫ�����Ȳ���ԭ��˵������������Ʒ����ɫ�������ָ�ԭ��˵�����������������2�֣�
�����������1���������������ԣ��ܰ�SO2����������������ᣬ��Ӧ�����ӷ���ʽ��Cl2+SO2+2H2O��4H++2Cl�D+SO42��������SO2������ˮ��Ҳ����ˮ��Ӧ�����ܽ�Ƚϴ�ᷢ����������Fװ�õ������Ƿ�ֹ������
��2������Cl2��������������Һ����ǿ�����ԣ��ܰ����������������������ӣ��ݴ˿���ͨ�����������������������Ƿ����������ѡb��c��d��
���������Ͷ���������Ư���ԣ���ѡ��Ʒ����������Cl2��������SO2����������ѡe����Ʒ����ɫ�����Ȳ���ԭ��˵������������Ʒ����ɫ�������ָ�ԭ��˵���������������
�����������Ǹ߿��еij�����������ͣ������е��Ѷ�����Ŀ��飬�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶�Ͻ���ʵ��������������ѧ����ѧ������������Ĺؼ�����ȷ���ʵ����ʼ������Ļ�ѧ��Ӧ������������ü��ɡ�

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ


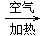 CuO
CuO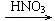 Cu��NO3��2
Cu��NO3��2 CuSO4
CuSO4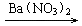 Cu��NO3��2
Cu��NO3��2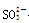 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�