��Ŀ����
������ѧ�������һ���˺��ձ���ѧ�Ҹ���Ӣһ����ľ�������з����л��ϳ��е��ٴ��Ľ���ż���������2010���ŵ������ѧ�����л��ϳɳ��õ���/����̿����������ʹ�ô����ᱻ���ʣ��磺�����л���ȣ���Ⱦ��ʧȥ���ԣ���Ϊ�ϴ�����������������գ�һ���ɷϴ�����ȡ�Ȼ��ٵĹ����������£�
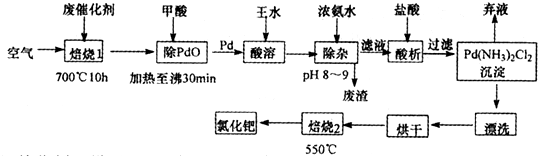
��1�����ٴ�������ɺ�����700��ĸ����±��գ����չ�������ͨ������������ԭ����______�����ỹԭ�����ٵĻ�ѧ����ʽΪ______��
��2��������ˮ��Ũ������Ũ���ᰴ�����1��3����ת��ΪH2PdCl4�����ỹԭΪNO���÷�Ӧ�Ļ�ѧ����ʽΪ______��
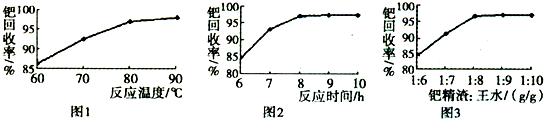
��3���پ������ٵĻ����ʸߵ���Ҫȡ������ˮ�ܽ�IJ�����������֪��Ӧ�¶ȡ���Ӧʱ�����ˮ�������ٻ����ʵ�Ӱ����ͼ1��ͼ3��ʾ������ˮ�ܽ��پ����������������¶ȡ�ʱ�����ˮ������Ϊ______��______��______��
��4����Ũ��ˮʱ����ת��Ϊ������[Pd��NH3��4]2+����ʱ���Ĵ�����ʽ��______��д��ѧʽ����
��5��700�決��1��Ŀ���ǣ�______��550�決��2��Ŀ���ǣ�______��

��1�����ٴ�������ɺ�����700��ĸ����±��գ����չ�������ͨ������������ԭ����______�����ỹԭ�����ٵĻ�ѧ����ʽΪ______��
��2��������ˮ��Ũ������Ũ���ᰴ�����1��3����ת��ΪH2PdCl4�����ỹԭΪNO���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3���پ������ٵĻ����ʸߵ���Ҫȡ������ˮ�ܽ�IJ�����������֪��Ӧ�¶ȡ���Ӧʱ�����ˮ�������ٻ����ʵ�Ӱ����ͼ1��ͼ3��ʾ������ˮ�ܽ��پ����������������¶ȡ�ʱ�����ˮ������Ϊ______��______��______��

��4����Ũ��ˮʱ����ת��Ϊ������[Pd��NH3��4]2+����ʱ���Ĵ�����ʽ��______��д��ѧʽ����
��5��700�決��1��Ŀ���ǣ�______��550�決��2��Ŀ���ǣ�______��
���ٴ�������ɺ�����700��ĸ����±��գ�Fe��Pd���л��ﱻ��������������������������м�����ᣬ�������������Ӧ�����κ�ˮ��PdO�ͼ��ᷢ��������ԭ��Ӧ����Pd�������к���Pd��SiO2���������費������ˮ����Pd��������ˮ������Һ��ͬʱ�������壬����Ũ��ˮ������ҺPH���������ӣ��õ���Һ���м������������õ�����Pd��NH3��2Cl2 ��ͨ���Ѱ���һϵ�в����õ��Ȼ��٣�
��1�����ٴ����к���̼���ʣ�̼��������Ӧ���ɶ�����̼��Ϊ��ʹ̼���ȼ�գ�Ҫͨ������������ٱ�������������PdO��PdO��HCOOH����������ԭ��Ӧ����Pd��CO2��H2O����Ӧ����ʽΪPdO+HCOOH=Pd+CO2��+H2O��
�ʴ�Ϊ��ʹ����̿���ȼ�ն���ȥ��PdO+HCOOH=Pd+CO2��+H2O��
��2���ڷ�Ӧ��PtԪ�صĻ��ϼ۱仯Ϊ0��+4��1��Ptԭ�ӵı仯��Ϊ4��NԪ�صĻ��ϼ۱仯Ϊ+5��+2��1��Nԭ�ӵı仯��Ϊ3����Ϊ��֤���ϼ���������ȣ�Ȼ�����Ptԭ�Ӹ�����ȣ���Nԭ�ӣ�Clԭ���غ㣬Ȼ���ٸ���ԭ���غ��������Ԫ�أ���3Pt+4HNO3+18HCl�T3H2PtCl6+4NO��+8H2O��
�ʴ�Ϊ��3Pt+4HNO3+18HCl�T3H2PtCl6+4NO��+8H2O��
��3������ͼ��֪���¶�Խ���ٻ�����Խ��Ӧʱ��Խ���ٻ�����Խ���پ�������ˮ��������Խ���ٻ�����Խ��90������ʱ�¶��ٸ��ٻ���������8h������ʱ����������ٻ����������پ�������ˮ��������Ϊ1��8���������پ�������ˮ�������ȣ����ٻ����ʲ�����������������80��90�棨��90�����ң�����Ӧʱ��ԼΪ8h���پ�������ˮ��������Ϊ1��8��
�ʴ�Ϊ��80��90�棨��90�����ң�����Ӧʱ��ԼΪ8h���پ�������ˮ��������Ϊ1��8��
��4����Ũ��ˮʱ����ת��Ϊ������[Pd��NH3��4]2+��������Һ�������ӱ�����Ϊ�����������ʴ�Ϊ��Fe��OH��3��
��5����700��ĸ����±��գ�C��Fe���л��ﱻ�������������������ȥ����̿���л��550�決��2��Ŀ�����Ѱ���Pd��NH3��2Cl2�仯ΪPdCl2��
�ʴ�Ϊ����ȥ����̿���л���Ѱ���Pd��NH3��2Cl2�仯ΪPdCl2��
��1�����ٴ����к���̼���ʣ�̼��������Ӧ���ɶ�����̼��Ϊ��ʹ̼���ȼ�գ�Ҫͨ������������ٱ�������������PdO��PdO��HCOOH����������ԭ��Ӧ����Pd��CO2��H2O����Ӧ����ʽΪPdO+HCOOH=Pd+CO2��+H2O��
�ʴ�Ϊ��ʹ����̿���ȼ�ն���ȥ��PdO+HCOOH=Pd+CO2��+H2O��
��2���ڷ�Ӧ��PtԪ�صĻ��ϼ۱仯Ϊ0��+4��1��Ptԭ�ӵı仯��Ϊ4��NԪ�صĻ��ϼ۱仯Ϊ+5��+2��1��Nԭ�ӵı仯��Ϊ3����Ϊ��֤���ϼ���������ȣ�Ȼ�����Ptԭ�Ӹ�����ȣ���Nԭ�ӣ�Clԭ���غ㣬Ȼ���ٸ���ԭ���غ��������Ԫ�أ���3Pt+4HNO3+18HCl�T3H2PtCl6+4NO��+8H2O��
�ʴ�Ϊ��3Pt+4HNO3+18HCl�T3H2PtCl6+4NO��+8H2O��
��3������ͼ��֪���¶�Խ���ٻ�����Խ��Ӧʱ��Խ���ٻ�����Խ���پ�������ˮ��������Խ���ٻ�����Խ��90������ʱ�¶��ٸ��ٻ���������8h������ʱ����������ٻ����������پ�������ˮ��������Ϊ1��8���������پ�������ˮ�������ȣ����ٻ����ʲ�����������������80��90�棨��90�����ң�����Ӧʱ��ԼΪ8h���پ�������ˮ��������Ϊ1��8��
�ʴ�Ϊ��80��90�棨��90�����ң�����Ӧʱ��ԼΪ8h���پ�������ˮ��������Ϊ1��8��
��4����Ũ��ˮʱ����ת��Ϊ������[Pd��NH3��4]2+��������Һ�������ӱ�����Ϊ�����������ʴ�Ϊ��Fe��OH��3��
��5����700��ĸ����±��գ�C��Fe���л��ﱻ�������������������ȥ����̿���л��550�決��2��Ŀ�����Ѱ���Pd��NH3��2Cl2�仯ΪPdCl2��
�ʴ�Ϊ����ȥ����̿���л���Ѱ���Pd��NH3��2Cl2�仯ΪPdCl2��

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ

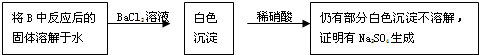


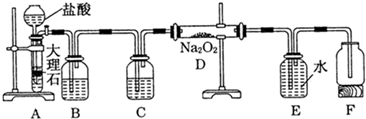
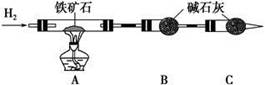
 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�