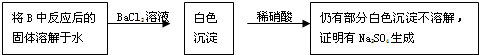
��Ŀ����
�������Ҫ�����Ʊ����࣬������ֲ�������ٽ�������Чɱ����������ȩ���������ڼ��Դ���������ˮK2CO3�������£���������ᣬ����ʽ���£�
C6H5CHO+��CH3CO��2O?C6H5CH=CHCOOH+CH3COOH
�й��������±���
ʵ������ȡ�����IJ������£�
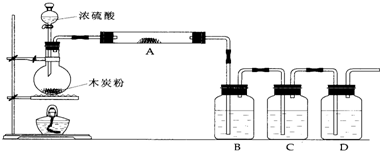
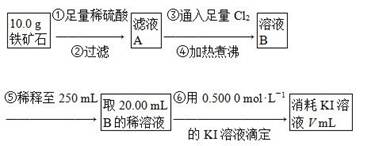
����250mL������ƿ�У�����7g��ˮK2CO3��5mL����ȩ��14mL���������������ȣ�������ԡ���ȣ�����45min����ͼһ�������ܿɲ�ͨ����ˮ����
���������20mLˮ������������������̼���ƣ�ʹ��Һ�����ԣ�����ˮ����������ͼ������ֱ������״������Ϊֹ��
��ˮ�����������Һ������������̿����������ӳ��ȹ��ˣ��ڽ�����������Һ�м���Ũ�����������ԣ�
����ȴ�����ˣ���������ˮϴ�Ӳ�������ڿ��������ɣ����أ�������ʣ�
�ش��������⣺
��1�����������Ʊ��У�ˮ���������Ŀ����______��
��2����������Ʊ�ʵ���У�ΪʲôҪ�����������̼����������pHֵ��______��
��3����������ˮϴ�Ӳ����Ŀ�ģ�______��
��4����Ũǿ������£�C6H5CHO������Ӧ��2C6H5CHO+KOH��Ũ����C6H5COOK+C6H5CH2OH��Ӧ����Ϊ��______��
��5��д������ȩ�ͱ�����[��CH3CH2CO��2O]����ˮK2CO3�����£������ķ�Ӧ�Ļ�ѧ����ʽ��______��
C6H5CHO+��CH3CO��2O?C6H5CH=CHCOOH+CH3COOH
�й��������±���
| ���� | ��CH3CO��2O | C6H5CHO | C6H5CH=CHCOOH | CH3COOH |
| �е� | 139�� | 179�� | 300�� | 117.9�� |
����250mL������ƿ�У�����7g��ˮK2CO3��5mL����ȩ��14mL���������������ȣ�������ԡ���ȣ�����45min����ͼһ�������ܿɲ�ͨ����ˮ����
���������20mLˮ������������������̼���ƣ�ʹ��Һ�����ԣ�����ˮ����������ͼ������ֱ������״������Ϊֹ��
��ˮ�����������Һ������������̿����������ӳ��ȹ��ˣ��ڽ�����������Һ�м���Ũ�����������ԣ�
����ȴ�����ˣ���������ˮϴ�Ӳ�������ڿ��������ɣ����أ�������ʣ�

�ش��������⣺
��1�����������Ʊ��У�ˮ���������Ŀ����______��
��2����������Ʊ�ʵ���У�ΪʲôҪ�����������̼����������pHֵ��______��
��3����������ˮϴ�Ӳ����Ŀ�ģ�______��
��4����Ũǿ������£�C6H5CHO������Ӧ��2C6H5CHO+KOH��Ũ����C6H5COOK+C6H5CH2OH��Ӧ����Ϊ��______��
��5��д������ȩ�ͱ�����[��CH3CH2CO��2O]����ˮK2CO3�����£������ķ�Ӧ�Ļ�ѧ����ʽ��______��
��1������ˮ��������ʹ�л�����ڽϵ͵��¶��´ӻ������������������Ա����ڳ�ѹ������ʱ����ɵ���ʧ����߷����ᴿ��Ч�ʣ�ͬʱ�ڲ�����װ�÷���Ҳ�ϼ�ѹ������һЩ������ˮ�����������Ӧ���ڷ�����ᴿ�л��
�ʴ�Ϊ��Ϊ����������ȩ��
��2����������Ʊ�ʵ���У������������̼���ƣ����Լ�С������̼�������ʣ������ڵ���PH�����Ի����������̼����������pHֵԭ���ǣ�������̼���Ƶ��ٶȹ��죬�ײ�������CO2�����ݣ����Ҳ�����ȷ����pHֵ��
�ʴ�Ϊ��������̼���Ƶ��ٶȹ��죬�ײ�������CO2�����ݣ����Ҳ�����ȷ����pHֵ��
��3������ϴ����Ϊ��ϴȥ�����������ʣ���ˮ�й����ܽ�ȼ�С�����Լ��ٹ�����ʧ��
�ʴ�Ϊ���ᴿ��ϴȥ���������������ӣ�ͬʱ����ˮ��Ϊ�˼��ٹ������ܽ��������ʧ��
��4��2C6H5CHO+KOH��Ũ����C6H5COOK+C6H5CH2OH��Ӧ�У�ȩ�������������Ȼ���ͬʱȩ������ԭ�ɴ��ǻ�������������ԭ��Ӧ��
�ʴ�Ϊ��������ԭ��Ӧ��
��5�����ݷ�ӦC6H5CHO+��CH3CO��2O?C6H5CH=CHCOOH+CH3COOH������ȩ�ͱ�����[��CH3CH2CO��2O]����ˮK2CO3�����·�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
�ʴ�Ϊ��Ϊ����������ȩ��
��2����������Ʊ�ʵ���У������������̼���ƣ����Լ�С������̼�������ʣ������ڵ���PH�����Ի����������̼����������pHֵԭ���ǣ�������̼���Ƶ��ٶȹ��죬�ײ�������CO2�����ݣ����Ҳ�����ȷ����pHֵ��
�ʴ�Ϊ��������̼���Ƶ��ٶȹ��죬�ײ�������CO2�����ݣ����Ҳ�����ȷ����pHֵ��
��3������ϴ����Ϊ��ϴȥ�����������ʣ���ˮ�й����ܽ�ȼ�С�����Լ��ٹ�����ʧ��
�ʴ�Ϊ���ᴿ��ϴȥ���������������ӣ�ͬʱ����ˮ��Ϊ�˼��ٹ������ܽ��������ʧ��
��4��2C6H5CHO+KOH��Ũ����C6H5COOK+C6H5CH2OH��Ӧ�У�ȩ�������������Ȼ���ͬʱȩ������ԭ�ɴ��ǻ�������������ԭ��Ӧ��
�ʴ�Ϊ��������ԭ��Ӧ��
��5�����ݷ�ӦC6H5CHO+��CH3CO��2O?C6H5CH=CHCOOH+CH3COOH������ȩ�ͱ�����[��CH3CH2CO��2O]����ˮK2CO3�����·�Ӧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
��
��ϰ��ϵ�д�
�����Ŀ