��Ŀ����
����Ŀ��NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ����NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش�����������
��1��NH4Al(SO4)2������ˮ������������_________________________(�ñ�Ҫ�Ļ�ѧ������������˵��)��
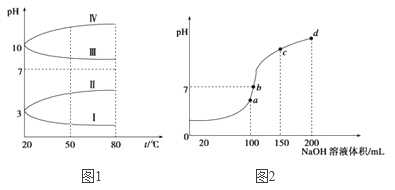
��2����ͼ1��0.1 mol��L-1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0.1 mol��L-1NH4Al(SO4)2��pH���¶ȱ仯��������_______________(��д��ĸ)������pH���¶ȱ仯��ԭ����________________��
��NH4Al(SO4)2������Һ��NH4HCO3������Һ���,��Ӧ�����ӷ���ʽΪ________________��(3)����ʱ����100 mL 0.1mol��L-1NH4HSO4��Һ�еμ�0.1mol��L-1NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶��ɴ�С˳��Ϊ��______________����b�㣬��Һ�и�����Ũ���ɴ�С������˳����__________��
��4����֪25����NH3��H2O��Kb=1.8��10-5��H2SO3��Ka1=1.3��10-2��K2=6.2��10-8����SO2ͨ��ð�ˮ�У���c(OH-)����1.0��10-7mol��L-1ʱ����Һ�е�c(SO32-)/c(HSO3-)=____________��
��5����25��ʱ��Ka(HCN)=4.9��10-10��Ka(CH3COOH)=1.8��10-5��H2CO3��K1=4.3��10-7��K2=5.6��10-11��25����pH ��ȵ�NaCN ��Һ��Na2CO3��Һ��CH3COONa ��Һ������Һ�����ʵ���Ũ���ɴ�С��˳��Ϊ_________________��
����NaCN ��Һ��ͨ������CO2 ����������Ӧ�Ļ�ѧ����ʽΪ��__________________��
���𰸡� A13+ˮ�����ɵ�Al(OH)3������������ԣ���Al3++3H2O=Al(OH)3(����)+3H+��Al(OH)3 ������������ʹ������Ӷ�����ˮ �� NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶ȣ���ˮ��̶����� pH ��С Al3++3HCO3-=Al(OH)3��+3CO2�� a>b>c>d c (Na+)>c(SO42-)>c(NH4+)>c(OH-)=c(H+) 0.62 CH3COONa ��Һ>NaCN ��Һ>Na2CO3��Һ NaCN+H2O+CO2=HCN+NaHCO3
����������1��.Al3+ˮ�����ɵ�Al(OH)3������������ԣ��ܹ�����ˮ�е���������ʹ������Ӷ��ﵽ��ˮ��Ŀ�ģ����ӷ���ʽΪ��Al3++3H2O=Al(OH)3(����)+3H+���ʴ�Ϊ��A13+ˮ�����ɵ�Al(OH)3������������ԣ���Al3++3H2O=Al(OH)3(����)+3H+��Al(OH)3������������ʹ������Ӷ�����ˮ��
��2��.��. NH4Al(SO4)2��ǿ�������Σ�ˮ��ʹ��Һ�����ԣ������¶ȴٽ�ˮ�⣬ʹ��ˮ��̶�����pH��С�����ϵ�����Ϊ�ʴ�Ϊ����NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶ȣ���ˮ��̶����� pH��С��
��.NH4Al(SO4)2������Һ��NH4HCO3������ҺҺ�����Al3����HCO3������˫ˮ�ⷴӦ����Ӧ����ʽΪ��Al3++3HCO3-=Al(OH)3��+3CO2�����ʴ�Ϊ��Al3++3HCO3-=Al(OH)3��+3CO2����
��3��. a��b��c��d�ĸ��㣬���ݷ�Ӧ�����Ĺ�ϵ��a��ʱNaOHǡ�ú�NH4HSO4�е�H+��ȫ��Ӧ����Һ��ֻ�У�NH4��2SO4��Na2SO4��b��c��d������Һ������NH3H2O��Ũ��Խ��Խ������NH4��2SO4���Դٽ�ˮ�ĵ��룬��NH3H2O����ˮ�ĵ��룬���a��b��c��d�ĸ��㣬ˮ�ĵ���̶��ɴ�С˳��Ϊ��a>b>c>d��b����Һ�����ԣ���Һ�к��У�NH4��2SO4��Na2SO4��NH3H2O���ֳɷ֣���a��ʱc��Na+��=c��SO42-������b��ʱc��Na+��>c��SO42-��������NԪ����SԪ�صĹ�ϵ�����Եó�c��SO42-��>c��NH4+������c(Na+)>c(SO42-)>c(NH4+)>c(OH-)=c(H+)���ʴ�Ϊ��a>b>c>d��c c(Na+)>c(SO42-)>c(NH4+)>c(OH-)=c(H+)��
��4��. 25������SO2ͨ��ð�ˮ�У���c(OH-)����1.0��10-7mol��L-1ʱ��c��H+��=1.0��10-7mol��L-1����H2SO3��Ka2=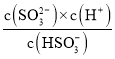 ��֪����Һ�е�c(SO32-)/c(HSO3-)=
��֪����Һ�е�c(SO32-)/c(HSO3-)=![]() =0.62���ʴ�Ϊ��0.62��
=0.62���ʴ�Ϊ��0.62��
��5��. ��.����ͼ�����ݿ�֪�����볣�������HCN��̼��������ӣ����ݡ�Խ��Խˮ�⡱����Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һˮ��̶�Ϊ��Na2CO3��Һ��NaCN��Һ��CH3COONa��Һ����Һ��pHΪ��Na2CO3��Һ��NaCN��Һ��CH3COONa��Һ����֮����pH��NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ�����ʵ���Ũ�ȴ�С˳��Ϊ��CH3COONa
��. ��NaCN��Һ��ͨ������CO2���������ԣ�H2CO3��HCN��HCO3-����Ӧ����HCN��̼�����ƣ��������ɶ�����̼���ʷ�Ӧ�Ļ�ѧ����ʽΪNaCN+H2O+CO2=HCN+NaHCO3���ʴ�Ϊ��NaCN+H2O+CO2=HCN+NaHCO3��

����Ŀ����1����0.lmol��L-1NaOH��Һ�ֱ�ζ������Ϊ20.00mL��Ũ�Ⱦ�Ϊ0.1mol��L-1������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߡ�
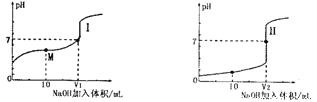
�ٵζ������������__________(�I����)��
��V1��V2�Ĺ�ϵ��V1______V2(�>������=����<��)
��M���Ӧ����Һ�У������ӵ����ʵ���Ũ���ɴ�С��˳����_______________��
��2��Ϊ���о������ܽ�ƽ��ͳ���ת����ijͬѧ�������ϲ��������ʵ�顣������AgSCN�ǰ�ɫ����
�������� | ���� |
����1����2mL0.005mol��L-1AgNO3��Һ�� ����2mL0.005mol��L-1KSCN��Һ������ | ���ְ�ɫ���� |
����2��ȡlmL�ϲ���Һ���Թ������μ�1 ��2 mol��L-1Fe(NO3)3��Һ | ��Һ���ɫ |
����3������2����Һ�У���������5��3mol��L-1 AgNO3��Һ | ���ְ�ɫ��������Һ��ɫ��dz |
����4������1���µ���Һ�м���5��3 mol��L-1 KI��Һ | ���ֻ�ɫ���� |
��д������2����Һ���ɫ�����ӷ���ʽ______________________��
���û�ѧƽ��ԭ�����Ͳ���3��ʵ������______________________��