��Ŀ����
��1��ij��ѧ��ȤС��Ҫ��ɷ�Ӧ�ȵIJⶨ��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�����������0��50mol�� L��1���ᡢ0��50mol�� L��1NaOH��Һ��ʵ����ȱ�ٵIJ�����Ʒ��_____________��_______________��
��2����֪2molCO������ȫȼ������CO2 ����ų�566 kJ������1 mol������ȫȼ������Һ̬ˮ�ų�286 kJ������1 molCH4������ȫȼ������CO2�����Һ̬ˮ�ų�890 kJ������д���ñ�ȼ������Ϊ��Ӧ�ȵ�COȼ�յ��Ȼ�ѧ����ʽ__________________��
��1 molCH4������ȫȼ������CO2�����Һ̬ˮ���ų�����_____890 kJ�� ������� ����������=����������a molCH4��CO��H2�Ļ��������ȫȼ�գ����� CO2�����Һ̬ˮ����CO2��ˮ�����ʵ������ʱ����ų�������Q���ĵ�ȡֵ��Χ��________________��
��1����Ͳ���¶ȼƣ���2�֣�
��2��CO (g)+1/2O2(g)=CO2 (g) ��H="-283" kJ��mol-1 ��2�֣�
> ��2�֣� 284��5a kJ<Q��586��5akJ ��2�֣�
�������������CO2����ת��Ϊ������ҲҪ�ų����������Էų�������890kJ,
CO (g)+1/2O2(g)=CO2(g) ��H="-283" kJ��mol-1 ---------------��1��
H2��g��+1/2 O2��g��=H2O��g����H="-286" kJ��mol-1-----------------��2��
CH4��g��+2 O2��g��=CO2��g��+2H2O ��g����H="-890" kJ��mol-1-------------------��3��
���ɵ�CO2��Һ̬H2O�����ʵ�����ȣ��ü�ֵ�������������¼��ֿ���
1������CO����������
2������H2�����ܣ�CO��CH4��Ϊa/2Ħ
�ų���������Q��=a/2��283+890��=586.5akJ
3������CH4������CO��H2��Ϊa/2Ħ
�ų���������Q��=a/2��283+286��=284.5akJ
�������������������
���Էų���������Q����ȡֵ��ΧΪ�� 284��5a kJ<Q��586��5akJ
���㣺�й��Ȼ�ѧ����ʽ��д�����㡣

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д� 2NO2(g);��H=" +57.0" kJ��mol-1��NO2��N2O4��Ũ����ͼ����ʾ��NO2��N2O4��������������Ũ�ȵĹ�ϵ����ͼ��ʾ��
2NO2(g);��H=" +57.0" kJ��mol-1��NO2��N2O4��Ũ����ͼ����ʾ��NO2��N2O4��������������Ũ�ȵĹ�ϵ����ͼ��ʾ��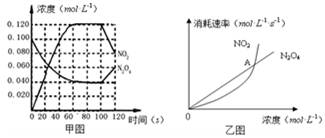
 CH3OH(g) ��H1
CH3OH(g) ��H1


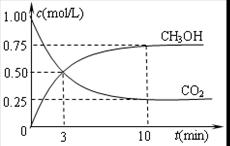
 SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��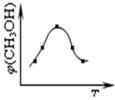
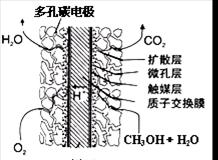
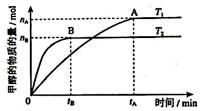
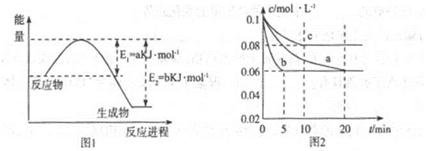
 cC(��)+dD(��)����H��Q������ͼ�ش�
cC(��)+dD(��)����H��Q������ͼ�ش�
 2NH3(g)��H<0
2NH3(g)��H<0