��Ŀ����
��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s) +O2 (g) == CO2(g) ��H1<0 ��
;��II�����Ƴ�ˮú����C(s) +H2O(g) == CO(g)+H2(g) ��H2>0 ��
��ȼ��ˮú����2CO(g)+O2 (g) == 2CO2(g) ��H3<0 ��
2H2(g)+O2 (g) == 2H2O(g) ��H4<0 ��
��ش��������⣺
��1�� ;��I�ų������� ( ����ڡ������ڡ���С�ڡ�) ;��II�ų���������
��2�� ��H1����H2����H3����H4����ѧ��ϵʽ�� ��
��3��12g̿���������в���ȫȼ������һ����̼���ų�110��35kJ���������Ȼ�ѧ����ʽΪ ��
��4��ú̿��Ϊȼ�ϲ���;��II���ŵ���
��1������ �� ��2����H1=��H2+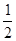 ����H3+��H4��
����H3+��H4��
��3��C(s) +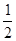 O2 (g) = CO(g) ��H=-110��35 kJ��mol-1
O2 (g) = CO(g) ��H=-110��35 kJ��mol-1
��4��ȼ��ȼ�ճ�֣������ʸߣ����ȶ࣬��ȾС��
��������������ɸ�˹����֪��Ӧ������;���أ�����;����ų�����������;����ų����������Ҳ���ȷ����H1����H2����H3����H4����ѧ��ϵʽ�ǡ�H1=��H2+1/2����H3+��H4��;
���㣺�Ȼ�ѧ����ʽ����д����˹���ɡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���������Ԫ���γɵij���������H2O��H2O2����һ�������¾��ɷֽ⡣
��1����֪��
| ��ѧ�� | �Ͽ�1mol��ѧ�������������kJ�� |
| H��H | 436 |
| O��H | 463 |
| O=O | 498 |
��H2O��g���ֽ���Ȼ�ѧ����ʽ�� ��
��11.2 L����״������H2��ȫȼ�գ�������̬ˮ���ų� kJ��������
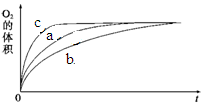
��2��ijͬѧ��H2O2�ֽ�Ϊ����̽��Ũ������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�����£����������ʾ�ķ������ʵ�顣
| ʵ���� | ��Ӧ�� | ���� | |
| a | 50 mL 5% H2O2��Һ | | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| b | 50 mL 5% H2O2��Һ | ����Ũ���� | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| c | 50 mL 5% H2O2��Һ | ����ŨNaOH��Һ | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| d | 50 mL 5% H2O2��Һ | | MnO2 |
ͼ1 ͼ2
�ɸ�ͼ�ܹ��ó���ʵ�������_________��
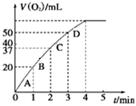
�ڲ��ʵ��d�ڱ�״���·ų������������ʱ��仯�Ĺ�ϵ��ͼ2��ʾ�����ͷ�Ӧ���ʱ仯��ԭ�� ��
��������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ɵõ������٣����ܷ�ӦΪ��
WO3 (s) + 3H2 (g) W (s) + 3H2O (g)
W (s) + 3H2O (g)
��ش��������⣺
��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___________________________��
��ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����H2��ƽ��ת����Ϊ_____________________�����¶ȵ����ߣ�H2��ˮ����������ȼ�С����÷�ӦΪ��Ӧ_____________________������ȡ����ȡ�����
�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
| �¶� | 25�� ~ 550�� ~ 600�� ~ 700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
��һ�η�Ӧ�Ļ�ѧ����ʽΪ___________________________��580��ʱ���������ʵ���Ҫ�ɷ�Ϊ________������WO3��ȫת��ΪW��������������H2���ʵ���֮��Ϊ____________________________________��
�� ��֪���¶ȹ���ʱ��WO2 (s)ת��ΪWO2 (g)��
WO2 (s) + 2H2 (g)
 W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1 WO2 (g) + 2H2(g)
 W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1
W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1 ��WO2 (s)
 WO2 (g) �Ħ�H �� ______________________��
WO2 (g) �Ħ�H �� ______________________������˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��W (s) +2I2 (g)
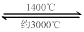 WI4 (g)������˵����ȷ����____________��
WI4 (g)������˵����ȷ����____________��a���ƹ��ڵ�I2��ѭ��ʹ��
b��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
c��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
d���¶�����ʱ��WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
 ��Һ���ܡ����������е�
��Һ���ܡ����������е�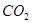 ��
��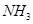 Ϊԭ�Ͽɺϳɻ�������[
Ϊԭ�Ͽɺϳɻ�������[ ]����֪��
]����֪��
 ��
��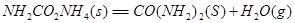
 ��
��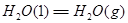
 ��
�� ���ڴ���������CO��
���ڴ���������CO�� ��Ӧ���ɼ״���
��Ӧ���ɼ״��� ij�ݻ��ɱ���ܱ������г���10molCO��20mol
ij�ݻ��ɱ���ܱ������г���10molCO��20mol
 _______VL��������ڡ�����С�ڡ����ڡ���
_______VL��������ڡ�����С�ڡ����ڡ���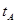 _______
_______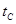 ���>������<����=����
���>������<����=���� 2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ�� 