��Ŀ����
��10�֣������dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ����Ҳ������ȡ������������ʹ�ñ���̼������Һ�нӡ��ش��������⣺
(1)����̼������Һ����Ҫ������ ��
(2)д���Ҵ������������ʵĻ�ѧ��Ӧ����ʽ
����ȡ�������� ��
��������Ʒ�Ӧ ��
�۴�����������ȩ ��
(3) �������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ��������������Ӧ�Ѵﵽ��ѧƽ��״̬����(�����)����������������
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
������Ӧ���������淴Ӧ���������
�ݻ�����и����ʵ�Ũ�����
��Ӧ���ټ�������
(1)����̼������Һ����Ҫ������ ��
(2)д���Ҵ������������ʵĻ�ѧ��Ӧ����ʽ
����ȡ�������� ��
��������Ʒ�Ӧ ��
�۴�����������ȩ ��
(3) �������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ��������������Ӧ�Ѵﵽ��ѧƽ��״̬����(�����)����������������
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
������Ӧ���������淴Ӧ���������
�ݻ�����и����ʵ�Ũ�����
��Ӧ���ټ�������
��1���кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��2��CH3COOH + CH3CH2OH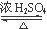 CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O
2CH3CH2OH+2Na��2CH3CH2ONa+H2 2CH3CH2OH+O2��2CH3CHO+H2O
��5���ڢ�
��2��CH3COOH + CH3CH2OH
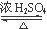 CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O 2CH3CH2OH+2Na��2CH3CH2ONa+H2 2CH3CH2OH+O2��2CH3CHO+H2O
��5���ڢ�
��1���������ɵ����������к���������Ҵ�������ͨ������̼������Һ����ȥ���ʡ����������кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��2���Ҵ������ǻ����ܷ���������Ӧ���ܺͽ����Ʒ�Ӧ����������Ҳ�ܷ���������������ȩ������ʽ�ֱ�ΪCH3COOH + CH3CH2OH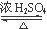 CH3COOCH2CH3 + H2O��
CH3COOCH2CH3 + H2O��
2CH3CH2OH+2Na��2CH3CH2ONa+H2��2CH3CH2OH+O2��2CH3CHO+H2O��
��3����һ�������£������淴Ӧ������Ӧ���ʺ��淴Ӧ�������ʱ������Ϊ0������Ӧ��ϵ�и������ʵ�Ũ�Ȼ������ٷ����仯��״̬����Ϊ��ѧƽ��״̬�����Ԣ���ȷ������ȷ���١����з�Ӧ���ʵķ�������ͬ�ģ�����ȷ�����з�Ӧ���ʵķ����෴������������֮������Ӧ�Ļ�ѧ������֮�ȣ���ȷ��ƽ��ʱŨ�Ȳ��ٷ����仯��������֮���Ũ�Ȳ�һ����Ȼ�����ij�ֹ�ϵ������ѡ��ݲ���ȷ����ѡ�ڢܡ�
��2���Ҵ������ǻ����ܷ���������Ӧ���ܺͽ����Ʒ�Ӧ����������Ҳ�ܷ���������������ȩ������ʽ�ֱ�ΪCH3COOH + CH3CH2OH
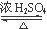 CH3COOCH2CH3 + H2O��
CH3COOCH2CH3 + H2O��2CH3CH2OH+2Na��2CH3CH2ONa+H2��2CH3CH2OH+O2��2CH3CHO+H2O��
��3����һ�������£������淴Ӧ������Ӧ���ʺ��淴Ӧ�������ʱ������Ϊ0������Ӧ��ϵ�и������ʵ�Ũ�Ȼ������ٷ����仯��״̬����Ϊ��ѧƽ��״̬�����Ԣ���ȷ������ȷ���١����з�Ӧ���ʵķ�������ͬ�ģ�����ȷ�����з�Ӧ���ʵķ����෴������������֮������Ӧ�Ļ�ѧ������֮�ȣ���ȷ��ƽ��ʱŨ�Ȳ��ٷ����仯��������֮���Ũ�Ȳ�һ����Ȼ�����ij�ֹ�ϵ������ѡ��ݲ���ȷ����ѡ�ڢܡ�

��ϰ��ϵ�д�
�����Ŀ
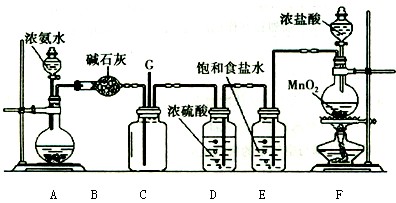
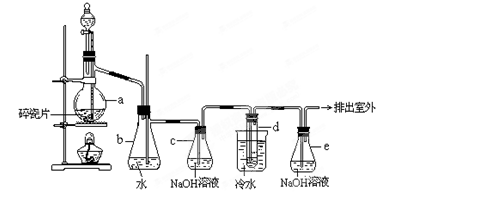
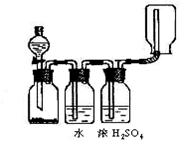
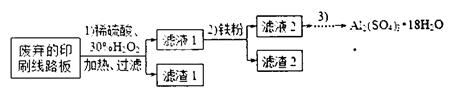
 4NO+6H2O�������ͼ��ѡ�����������(���ظ�ʹ��)���һ���и÷�Ӧ�ļ�װ�á����ṩ�Լ����������ơ���ʯ�ҡ����ۡ��Ȼ��ơ�Ũ���ᡢŨ��ˮ������������Һ��
4NO+6H2O�������ͼ��ѡ�����������(���ظ�ʹ��)���һ���и÷�Ӧ�ļ�װ�á����ṩ�Լ����������ơ���ʯ�ҡ����ۡ��Ȼ��ơ�Ũ���ᡢŨ��ˮ������������Һ��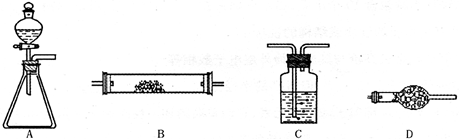

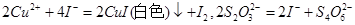
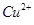 �������� ���ƫ�ߡ�����ƫ
�������� ���ƫ�ߡ�����ƫ
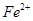 ��ȥ���±��г��˼������������������������pH����ʼ������pH����������Ũ��Ϊ1��0mo1��L-1���㣩��
��ȥ���±��г��˼������������������������pH����ʼ������pH����������Ũ��Ϊ1��0mo1��L-1���㣩�� ������ȡCO��
������ȡCO��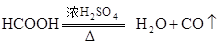
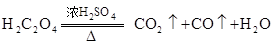
 ��CO����?
��CO����?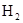 �Ļ�ԭ��˭ǿ?
�Ļ�ԭ��˭ǿ?