��Ŀ����
����Ŀ������������ȷ����
A.�����£�pHֵ����14��pHֵ����12������NaOH��Һ�������ͺ�c��H������(10��14��10��10)/2
B.�����£�Ũ��Ϊ1��10��10mol/L��KOH��Һ��pHֵ��ӽ���4
C.��ˮ��ˮϡ�ͣ���Һ�г�ˮ������������ӵ�Ũ�ȶ���С
D.�����£���ͬ�¶���pHֵ����1��������Һ��ˮ�ĵ���̶���pHֵ����13��Ba(OH)2��Һ��ˮ�ĵ���̶����
���𰸡�D
��������
A.pH=14��NaOH��Һ��c(OH��)=1mol��L��1��pH=12��NaOH��Һc(OH��)=10��2mol��L��1�����ߵ������Ϻ�c(OH��)=![]() mol��L��1������ˮ�����ӻ���c(H��)=
mol��L��1������ˮ�����ӻ���c(H��)=![]() mol��L��1����A����
mol��L��1����A����
B.KOH����ǿ���Һ�ʼ��ԣ�������Ũ��Ϊ1��10��10mol/L��KOH��Һ��pH��ӽ���7����B����
C.��ˮ��ˮϡ�ͣ��ٽ�NH3��H2O�ĵ��룬c(NH3��H2O)��c(OH��)��c(NH4��)��С��c(H��)Ũ������C����
D.������Һ��OH-ȫ������ˮ�ĵ��룬Ba��OH��2��Һ��H+ȫ������ˮ�ĵ��룬�����£�pH=1��������Һ��ˮ�������c(OH-)=Kw/c(H��)=10��13mol/L��pH=13��Ba(OH)2��Һ��ˮ�������c(H��)=10��13mol/L�����ˮ�ĵ���̶���ȣ���D��ȷ��
��ѡD��

����Ŀ����֪![]() �ڵ����±Ƚ��ȶ������Աȴ�����ǿ���������������л�ԭ�ԣ������������ԭ��������ҺpH�Ĺ�ϵ�����
�ڵ����±Ƚ��ȶ������Աȴ�����ǿ���������������л�ԭ�ԣ������������ԭ��������ҺpH�Ĺ�ϵ�����
pH��Χ |
|
|
���� |
| NO�� |
�����й�˵���������![]()
A.���������£�![]() ��NaClO��Ӧ�����ӷ���ʽΪ
��NaClO��Ӧ�����ӷ���ʽΪ![]()
B.�����![]() ��Һ��ͨ��
��Һ��ͨ��![]() �ɵõ�
�ɵõ�![]()
C.�����![]() ��Һ�м���ϡ����ɵõ�
��Һ�м���ϡ����ɵõ�![]()
D.�����![]() ��Һ�м�����е��۵�����ᣬ��Һ����ɫ
��Һ�м�����е��۵�����ᣬ��Һ����ɫ
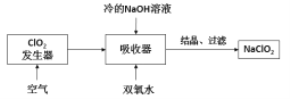
����Ŀ�����Ȼ���������ýȾ����������ͼ��ʾװ�ÿ����Ʊ����Ȼ��������ּг�װ������ȥ����

�й���Ϣ���±���
��ѧʽ | SnCl2 | SnCl4 |
�۵�/�� | 246 | -33 |
�е�/�� | 652 | 144 |
�������� | ��ɫ���壬������ | ��ɫҺ�壬��ˮ�� |
�ش��������⣺
��1����װ��������A������Ϊ___________��
��2���ü�װ����������MnO4- ����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ________________��
��3����װ����ͼ���Ӻã���������ԣ���������Ũ���ᣬ���۲쵽__________��������ʼ���ȶ�װ�ã����ۻ����ʵ����������������������ȶ�װ�ã���ʱ�������ȶ�װ�õ�Ŀ���ǣ�
�ٴٽ�����������Ӧ��
��_______________________________��
��4�����ȱ����װ�ã����ܷ����ĸ���Ӧ�Ļ�ѧ����ʽΪ___________________����װ�õ�������_________________��
A.��ȥδ��Ӧ����������ֹ��Ⱦ����
B.��ֹ������CO2���������װ��
C.��ֹˮ����������װ�õ��Թ���ʹ����ˮ��
D.��ֹ������O2������װ�õ��Թ���ʹ��������
��5��ijͬѧ��Ϊ��װ���еķ�Ӧ���ܲ���SnCl2���ʣ������Լ��п����ڼ���Ƿ����SnCl2 ����_______________�����ţ���
A. H2O2��Һ B. FeCl3��Һ������KSCN�� C. AgNO3��Һ D. ��ˮ
��6����Ӧ����ȥ����1.19g����Ӧ������װ�õ��Թ����ռ���2.38gSnCl4����SnCl4�IJ���Ϊ________��������3λ��Ч���֣�
����Ŀ�������£�������������Һ��
�� | �� | �� | �� | �� |
0.1 mol��L-1 CH3COOH��Һ | 0.01mol��L-1 CH3COOH��Һ | pH��2 CH3COOH��Һ | 0.1 mol��L-1 NaOH��Һ | 0.1mol��L-1 ��ˮ |
�ش��������⣺
��1����Һ��ϡ�͵�ԭ����10�������ҺpH______����Һ��pH������������������������������ͬ�����ٺ͢�����Һ��ˮ�������c(H��)����_______��
��2������ͬ�¶�ʱ100mL �ڵ���Һ��10mL �ٵ���Һ��Ƚϣ�������ֵǰ�ߴ��ں��ߵ���_______
A �к�ʱ����NaOH���� B ����̶�
C ˮ�������c(H��) D CH3COOH�����ʵ���
��3����ˮϡ�͢�ʱ����Һ������ˮ�������Ӷ���С����______������ĸ����
A��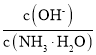 �������������� B��
�������������� B��
C��c��H+����c��OH-���ij˻���������D�� OH-�����ʵ���
��4��������N2H4������ˮ�Լ��ԣ���ԭ���백���ƣ�������Բ��簱ǿ��д��������ˮ�ʼ��Ե����ӷ���ʽ��________��