��Ŀ����
����Ŀ������(Ni)��������Լ20%���ϣ���Ҫ�ɷ��������Ͻ𣬻�����ͭ���ơ�þ�����������ɸ÷����Ʊ����Ƚϸߵ����������������������£�
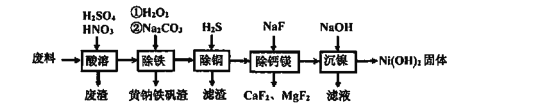
�ش��������⣺
(1)�Ͻ��е���������ϡ���ᣬ��������ʱ���˼���ϡ���ᣬ��Ҫ�߽����������ϡ���ᣬ��Ӧ��N2���ɡ�д���������ܽ�Ļ�ѧ����ʽ______________________
(2)��������ʱH2O2��������________________��Ϊ��֤�����ӵ�H2O2��������Ӧѡ����Լ���_______________ (����ţ��������軯��K3[Fe(CN)6]�����������軯��KSCN��)��Һ����������[NaxFey(SO4)m(OH)n]���г��������������ʿ졢�����˵��ص㣬��x��y��m��n=1��____��2��6
(3)����ͭ��ʱ����Ӧ�����ӷ���ʽΪ________________
(4)��֪Ksp(MgF2)=7.35��10-11��Ksp(CaF2)=1.05��10-10�����������NaF������þ����������Һ��![]() ________________(����1λС��)����֪���ӹ������մ������н��У�NaF��ʵ���������˹����ԭ����__________
________________(����1λС��)����֪���ӹ������մ������н��У�NaF��ʵ���������˹����ԭ����__________
(5)100kg���Ͼ����������Ƶ�Ni(OH)2���������Ϊ31kg������������Ϊ______________(����1λС��)
(6)�������ѳ�Ϊ��϶�����������Ҫ������ͣ��乤��ԭ�����£�M+Ni(OH)2![]() MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ___________
MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ___________
���𰸡�5Ni+5H2SO4+2HNO3=5NiSO4+N2��+6H2O ��Fe2+��������Fe3+ �� 3 H2S+Cu2+=CuS��+2H+ 0.7 ������F-�����������»ḯʴ�մ����� 98.3% Ni(OH)2+OH-��e-=NiOOH+H2O
��������
������������Լ20%�ķ��ϣ���Ҫ�ɷ��������Ͻ𣬻�����ͭ���ơ�þ��������������������������ܣ��Ͻ��е���������ϡ���ᣬ��������ʱ���˼���ϡ���ᣬ��Ҫ�߽����������ϡ���ᣬ�ܽ�Ni��Ӧ��N2���ɣ����˳�ȥ��������Һ�м����������������������Ϊ�����ӣ�����̼���Ƶ�����ҺpH��ȥ�����ӣ����˵õ���������Һ����Һ�м���H2S����ͭ���ӣ����˵õ���Һ�м���NaF������ȥþ���Ӻ����ӣ����˵õ���Һ����Ҫ�������ӣ���������������Һ�������������������������塣
��1���������ᷴӦ���������ӡ�������ˮ�����ԭ���غ㡢�����غ���ƽ��д��ѧ����ʽ��
��2����������������������Ϊ�����ӣ����ڳ�ȥ������Na2CO3��ȥFe3+�������������������ӣ��������軯�غ��������ӽ��������ɫ��Һ�������������Ƿ���ȫ�������������Ϣ����������[NaxFey��SO4��m��OH��n]Ԫ�ػ��ϼ۴�����Ϊ0��
��3�������ͭ���ӷ�Ӧ���������������ͭ������
��4�������ܶȻ�������Һ��Mg2+��Ca2+��Ũ��֮�ȣ�NaF���������˹�������Ϊ�����������ƻ���������Һ�����ɷ����⣬�մ������еĶ��������ͷ����ⷴӦ��
��5�����Ϻ�����������Լ20%��100kg���Ͼ����������Ƶ�Ni��OH��2���������Ϊ31kg��������Ԫ���غ������յõ�����ԭ��������������������ʣ�
��6��������Ni��OH��2ʧ��������NiOOH��
������������Լ20%�ķ��ϣ���Ҫ�ɷ��������Ͻ𣬻�����ͭ���ơ�þ��������������������������ܣ��Ͻ��е���������ϡ���ᣬ��������ʱ���˼���ϡ���ᣬ��Ҫ�߽����������ϡ���ᣬ�ܽ�Ni��Ӧ��N2���ɣ����˳�ȥ��������Һ�м����������������������Ϊ�����ӣ�����̼���Ƶ�����ҺpH��ȥ�����ӣ����˵õ���������Һ����Һ�м���H2S����ͭ���ӣ����˵õ���Һ�м���NaF������ȥþ���Ӻ����ӣ����˵õ���Һ����Ҫ�������ӣ���������������Һ�������������������������塣
��1���������ᷴӦ���������ӡ�������ˮ�����ԭ���غ㡢�����غ���ƽ��д��ѧ����ʽΪ��5Ni+5H2SO4+2HNO3=5NiSO4+N2��+6H2O��
�ʴ�Ϊ��5Ni+5H2SO4+2HNO3=5NiSO4+N2��+6H2O��
��2����������ʱH2O2�������ǣ���������������������Ϊ�����ӣ����ڳ�ȥ��Ϊ��֤�����ӵ�H2O2��������Ӧѡ����Լ����������軯�غ��������ӽ��������ɫ��Һ�������������Ƿ���ȫ�������ټ���Na2CO3ʹFe3+���ɻ�����������ȥ����������[NaxFey��SO4��m��OH��n]����Ԫ�ػ��ϼ�Ϊ+3�ۣ�Ԫ�ػ��ϼ۴�����Ϊ0��x+3y-2m-n=0���õ�x+3y=2m+n��x��y��m��n=1��p��2��6����p=3��
�ʴ�Ϊ����������������Ϊ�����ӣ��٣�3��
��3�������ͭ���ӷ�Ӧ���������������ͭ��������Ӧ�����ӷ���ʽΪ��H2S+Cu2+=CuS��+2H+��
�ʴ�Ϊ��H2S+Cu2+=CuS��+2H+��
��4��������Һ��![]() =
=![]() =
=![]() =
=![]() =0.7��NaF���������˹�������Ϊ�����������ƻ���������Һ�����ɷ����⣬�մ������еĶ��������ͷ����ⷴӦ�����������ḯʴ�մ�������
=0.7��NaF���������˹�������Ϊ�����������ƻ���������Һ�����ɷ����⣬�մ������еĶ��������ͷ����ⷴӦ�����������ḯʴ�մ�������
�ʴ�Ϊ��0.7��������F-�������������ḯʴ�մ�������
��5�����Ϻ�����������Լ20%��100kg���Ͼ����������Ƶ�Ni��OH��2���������Ϊ31kg����������Ԫ������=100kg��20%=20kg����Ӧ������Ԫ������=31��93��59kg�����������ʵļ���ʽ=(31��93��59)kg��20kg��100%=98.3%��
�ʴ�Ϊ��98.3%��
��6�������س��ʱ�ķ�ӦΪM+Ni��OH��2=MH+NiOOH���������������ĵ缫��Ӧʽ��Ni(OH)2+OH--e-=NiOOH+H2O���ʴ�Ϊ��Ni(OH)2+OH--e-=NiOOH+H2O��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�����Ŀ����1�����ײ��϶������ѣ�TiO2�����кܸߵĻ�ѧ���ԣ��������������Ĵ�������ҵ�϶������ѵ��Ʊ��ǣ�
���Ͽ�Ƭ | ||
���� | �۵� | �е� |
SiCl4 | -70�� | 57.6�� |
TiCl4 | -25�� | 136.5�� |
I. �������Ľ��ʯ����Ҫ�ɷ�TiO2����Ҫ����SiO2����̼�ۻ��װ���Ȼ�¯�У��ڸ�����ͨ��Cl2��Ӧ�Ƶû���SiCl4���ʵ�TiCl4��
II. ��SiCl4���룬�õ�������TiCl4��
III. ��TiCl4�м�ˮ�����ȣ�ˮ��õ�����TiO2��xH2O��
IV. TiO2��xH 2O���·ֽ�õ�TiO2��
��TiCl4��SiCl4�ڳ����µ�״̬��________��II������ȡ�IJ���������_______��
��III�з�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
����IV��ʵ������ɣ�Ӧ��TiO2��xH2O����________����������ţ��м��ȡ�

��2�����ݷ�ˮ�������к����ʵIJ�ͬ����ҵ���ж��ַ�ˮ�Ĵ���������

�ٷ�ˮI������CO2���������ӷ���ʽ��________________��
�ڷ�ˮ��������������ʵ���з��ַ�ˮ�е�c(HCO![]() )Խ��ˮЧ��Խ�ã�������Ϊ______________��
)Խ��ˮЧ��Խ�ã�������Ϊ______________��
�۷�ˮIII�еĹ�Ԫ�ش�������ת�����ڿո�������Ӧ�Ļ�ѧʽ����Hg2++______=CH3Hg++H+���ҹ��涨��Hg2+���ŷű����ܳ���0.05 mg��L����ij�����ŷŵķ�ˮ1 L�к�Hg2+ 3��10-7mo1���Ƿ�ﵽ���ŷű�__����ǡ�����
�ܷ�ˮ������C12����CN����CO2��N2�����μӷ�Ӧ��C12 ��CN-�����ʵ���֮��Ϊ5�U2����÷�Ӧ�����ӷ���ʽΪ__________��






