题目内容
(17分)I.为探究SO2的性质,需要标准状况下11.2 LSO2气体。化学小组同学依据化学方程式Zn+ 2H2SO4(浓)=ZnSO4+SO2↑+2H2O计算后,取32.5g锌粒与质量分数为98%的浓硫酸(密度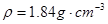 ) 60mL充分反应,锌全部溶解,对于制得的气体,有同学认为可能混有杂质。
) 60mL充分反应,锌全部溶解,对于制得的气体,有同学认为可能混有杂质。
(1)化学小组所制得的气体中混有的主要杂质气体可能是 (填分子式)。产生这种结果的主要原因是 (用化学方程式和必要的文字加以说明)
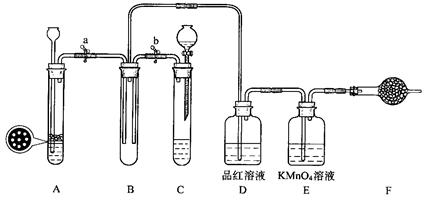
(2)为证实相关分析,化学小组的同学设计了实验,组装了如下装置,对所制取的气体进行探究。
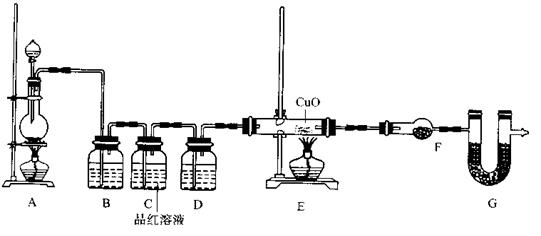
①置B中加入的试剂 ,装置C中品红溶液的作用是 。
②装置D加入的试剂 ,装置F加入的试剂 。
③可证实一定量的锌粒和一定量的浓硫酸反应后生成的气体中混有某杂质气体的实验现象是 。
④U型管G加入的试剂 ,作用为 .
II.工业上可采用电化学法利用H2S废气制取氢气,该法制氢过程的示意图所示,回答下列问题:
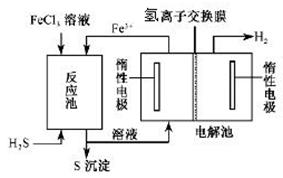
(1)产生反应池中发生反应的化学方程式为 。
(2)反应后的溶液进入电解池,电解时阳极反应式为 。
(3)若电解池中生成5. 6 L H2(标准状况),则理论上在反应池中可生成S沉淀的物质的量为___________ mol。
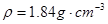 ) 60mL充分反应,锌全部溶解,对于制得的气体,有同学认为可能混有杂质。
) 60mL充分反应,锌全部溶解,对于制得的气体,有同学认为可能混有杂质。(1)化学小组所制得的气体中混有的主要杂质气体可能是 (填分子式)。产生这种结果的主要原因是 (用化学方程式和必要的文字加以说明)
(2)为证实相关分析,化学小组的同学设计了实验,组装了如下装置,对所制取的气体进行探究。

①置B中加入的试剂 ,装置C中品红溶液的作用是 。
②装置D加入的试剂 ,装置F加入的试剂 。
③可证实一定量的锌粒和一定量的浓硫酸反应后生成的气体中混有某杂质气体的实验现象是 。
④U型管G加入的试剂 ,作用为 .
II.工业上可采用电化学法利用H2S废气制取氢气,该法制氢过程的示意图所示,回答下列问题:

(1)产生反应池中发生反应的化学方程式为 。
(2)反应后的溶液进入电解池,电解时阳极反应式为 。
(3)若电解池中生成5. 6 L H2(标准状况),则理论上在反应池中可生成S沉淀的物质的量为___________ mol。
I.(1)H2(1分);随着反应的进行,硫酸浓度降低,致使锌与稀硫酸反应生成H2,
Zn+H2SO4===ZnSO4+H2↑(2分)
(2)①NaOH溶液(或KMnO4,其它合理答案也给分,1分)
证明SO2已被完全除尽。(1分)
②浓硫酸(1分) 无水硫酸铜(1分)
③装置E中玻璃管中黑色CuO粉末变红色,干燥管F中无水硫酸铜变蓝色(2分)
④碱石灰(1分)防止空气中H2O进入干燥管而影响杂质气体的检验(1分)
II. (1)H2S + 2FeCl3 = 2FeCl2 + S↓ + 2HCl(2分)
(2)2Fe2+ — 2e- 2Fe3+ (2分)
2Fe3+ (2分)
(3)0.25(2分)
Zn+H2SO4===ZnSO4+H2↑(2分)
(2)①NaOH溶液(或KMnO4,其它合理答案也给分,1分)
证明SO2已被完全除尽。(1分)
②浓硫酸(1分) 无水硫酸铜(1分)
③装置E中玻璃管中黑色CuO粉末变红色,干燥管F中无水硫酸铜变蓝色(2分)
④碱石灰(1分)防止空气中H2O进入干燥管而影响杂质气体的检验(1分)
II. (1)H2S + 2FeCl3 = 2FeCl2 + S↓ + 2HCl(2分)
(2)2Fe2+ — 2e-
 2Fe3+ (2分)
2Fe3+ (2分) (3)0.25(2分)
试题分析:I.(1)Zn的物质的量为0.5mol,浓硫酸中含H2SO4为1.84g/mL×60mL×98%÷98g/mol="1.1mol," 随着反应的进行,硫酸浓度降低,当硫酸变为稀硫酸时,发生反应:Zn+H2SO4===ZnSO4+H2↑,所以化学小组所制得的气体中混有的主要杂质气体可能是H2。
(2)①为了防止SO2对H2检验的干扰,装置B中应加入吸收SO2的试剂,NaOH溶液或KMnO4溶液等;装置C中品红溶液的作用是证明SO2已被完全除尽。
②H2还原CuO生成H2O,为了避免原气体中水分的干扰,装置D加入的试剂为浓硫酸;F的作用是检验气体与CuO反应生成了H2O,所以加入的试剂为无水硫酸铜。
③H2还原CuO的实验现象是:装置E中玻璃管中黑色CuO粉末变红色,干燥管F中无水硫酸铜变蓝色。
④为防止外界空气中H2O进入干燥管而影响杂质气体氢气的检验,装置G中应加入碱石灰。
II. (1)根据示意图,反应池加入的FeCl3与H2S为反应物,生成了S,发生了氧化还原反应,所以化学方程式为:H2S + 2FeCl3 = 2FeCl2 + S↓ + 2HCl
(2)反应后的溶液FeCl2和HCl进入溶液,生成H2的电极为阴极,所以阳极上失去电子的离子为Fe2+,所以阳极反应式为:2Fe2+ — 2e-
 2Fe3+
2Fe3+ (3)根据电解池中电子守恒和反应池的化学方程式可得关系式:S ~ 2Fe2+ ~ H2,所以n(S)=n(H2)=5.6L÷22.4L/mol=0.25mol。

练习册系列答案
相关题目
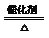 2SO3
2SO3
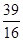 a% c.
a% c.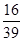 (1-a%) D.无法计算
(1-a%) D.无法计算