��Ŀ����
��ҵ�����������Ҫ��Ӧ���£�
4FeS2+11O2����2Fe2O3+8SO2 2SO2+O2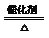 2SO3
2SO3
SO3+H2O=H2SO4��
�û����������ȡH2SO4������H2SO4������ȡ����(NH4)2SO4�����պ� FeS2 80.0% �Ļ����� 80.0 t������������ 83.6 t (NH4)2SO4����֪ NH3��������Ϊ 92.6%��H2SO4��������Ϊ 95.0%�������������ȡH2SO4ʱ����ʧ�ʡ�
4FeS2+11O2����2Fe2O3+8SO2 2SO2+O2
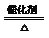 2SO3
2SO3SO3+H2O=H2SO4��
�û����������ȡH2SO4������H2SO4������ȡ����(NH4)2SO4�����պ� FeS2 80.0% �Ļ����� 80.0 t������������ 83.6 t (NH4)2SO4����֪ NH3��������Ϊ 92.6%��H2SO4��������Ϊ 95.0%�������������ȡH2SO4ʱ����ʧ�ʡ�
37.5%
��������� H2SO4���������� FeS2�����ʵĹ�ϵ�����ų� NH3�����ʵĸ������á�
��Σ����� S ԭ���غ��ҳ���֪�� FeS2��δ֪��(NH4)2SO4�Ĺ�ϵ(��������������Ϊx)��
FeS2 ~ 2H2SO4 ~ 2(NH4)2SO4
120 264
80.0 t��80.0%��95.0%��x 83.6 t
x=62.5%
���������ʧ��Ϊ��1.00��62.5%=37.5%��
ע�� (1)ԭ��������(��ת����) = ʵ��Ͷ��ԭ����������ת��ԭ������ ��100%
(2)������� = ��100%
��100%
(3)�м����������(��ת���ʡ�������)���ɹ�Ϊ��ʼԭ�ϵ������(��ת���ʡ�������)
(4)Ԫ�ص���ʧ��=�û��������ʧ��
��Σ����� S ԭ���غ��ҳ���֪�� FeS2��δ֪��(NH4)2SO4�Ĺ�ϵ(��������������Ϊx)��
FeS2 ~ 2H2SO4 ~ 2(NH4)2SO4
120 264
80.0 t��80.0%��95.0%��x 83.6 t
x=62.5%
���������ʧ��Ϊ��1.00��62.5%=37.5%��
ע�� (1)ԭ��������(��ת����) = ʵ��Ͷ��ԭ����������ת��ԭ������ ��100%
(2)������� =
 ��100%
��100%(3)�м����������(��ת���ʡ�������)���ɹ�Ϊ��ʼԭ�ϵ������(��ת���ʡ�������)
(4)Ԫ�ص���ʧ��=�û��������ʧ��

��ϰ��ϵ�д�
�����Ŀ
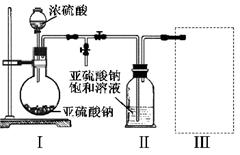

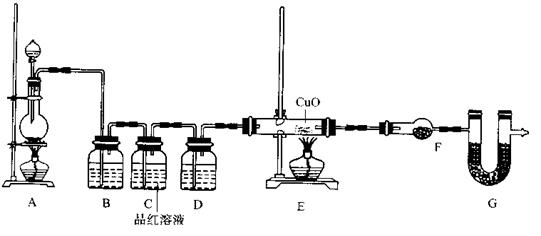
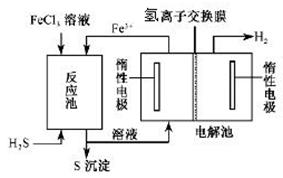
 Fe2O3��SO2����SO3�����������ɵ�����ͨ���Ȼ�����Һ�У��õ��ij�������( )
Fe2O3��SO2����SO3�����������ɵ�����ͨ���Ȼ�����Һ�У��õ��ij�������( ) �� 60mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
�� 60mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�