��Ŀ����
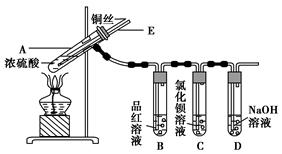
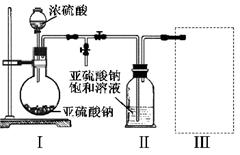
ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ʵ�飬ʵ��װ����ͼ��ʾ��
ʵ�鲽�裺
����������ͼ��ʾ��װ�ã����������ԣ��ټ����Լ���
�ڼ���A�Թܣ���B�Թ���Ʒ����Һ��ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿���ϳ鶯�뿪Һ�档
��ش��������⣺
(1)A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)�ܹ�֤��ͭ��Ũ���ᷴӦ���������ʵ�������� ��
(3)��ʢ��BaCl2��Һ��C�Թ��У����˵��ܿ��������⣬���������������������е���Һ�ֳ����ݣ��ֱ�μ�������Һ�������������Ļ�ѧʽ������ж�Ӧ��λ�á�
д������SO2���ֻ�ԭ�Ե����ӷ�Ӧ����ʽ�� ��
(4)ʵ����Ϻ���Ϩ��ƾ��ƣ����ڵ���E�Ĵ��ڣ��Թ�B�е�Һ�岻�ᵹ�����Թ�A�У���ԭ���� ��
(5)ʵ����Ϻ�װ���в����������ж������ܴ����ϵĽ�����Ϊ�˷�ֹ�����������������Ⱦ���������װ��ǰ��Ӧ����ȡ�IJ����� ��
(6)��SO2����ͨ�뺬��n mol Na2S����Һ�У���ַ�Ӧ����Һ�г��ֻ�ɫ���ǣ��Է�������Һ���������SO2���� mol(�������ܽ��SO2)��

ʵ�鲽�裺
����������ͼ��ʾ��װ�ã����������ԣ��ټ����Լ���
�ڼ���A�Թܣ���B�Թ���Ʒ����Һ��ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿���ϳ鶯�뿪Һ�档
��ش��������⣺
(1)A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)�ܹ�֤��ͭ��Ũ���ᷴӦ���������ʵ�������� ��
(3)��ʢ��BaCl2��Һ��C�Թ��У����˵��ܿ��������⣬���������������������е���Һ�ֳ����ݣ��ֱ�μ�������Һ�������������Ļ�ѧʽ������ж�Ӧ��λ�á�
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | | |
д������SO2���ֻ�ԭ�Ե����ӷ�Ӧ����ʽ�� ��
(4)ʵ����Ϻ���Ϩ��ƾ��ƣ����ڵ���E�Ĵ��ڣ��Թ�B�е�Һ�岻�ᵹ�����Թ�A�У���ԭ���� ��
(5)ʵ����Ϻ�װ���в����������ж������ܴ����ϵĽ�����Ϊ�˷�ֹ�����������������Ⱦ���������װ��ǰ��Ӧ����ȡ�IJ����� ��
(6)��SO2����ͨ�뺬��n mol Na2S����Һ�У���ַ�Ӧ����Һ�г��ֻ�ɫ���ǣ��Է�������Һ���������SO2���� mol(�������ܽ��SO2)��
(1)Cu��2H2SO4(Ũ)  CuSO4��SO2����2H2O
CuSO4��SO2����2H2O
(2)B�Թ���Ʒ����Һ��ɫ
(3)BaSO4��BaSO3��SO2��Cl2��2H2O=4H����SO42-��2Cl��(��Ba2����SO2��Cl2��2H2O=BaSO4����4H����2Cl��)
(4)��A�Թ�������ѹǿ��Сʱ��������E���ܽ���A�Թ��У�ά��A�Թ���ѹǿƽ��
(5)��E���ܿ���A�Թ��л����ع��������Ŀ�������������SO2�������NaOH��Һ�У�ʹ֮����ȫ����
(6)2.5n
 CuSO4��SO2����2H2O
CuSO4��SO2����2H2O(2)B�Թ���Ʒ����Һ��ɫ
(3)BaSO4��BaSO3��SO2��Cl2��2H2O=4H����SO42-��2Cl��(��Ba2����SO2��Cl2��2H2O=BaSO4����4H����2Cl��)
(4)��A�Թ�������ѹǿ��Сʱ��������E���ܽ���A�Թ��У�ά��A�Թ���ѹǿƽ��
(5)��E���ܿ���A�Թ��л����ع��������Ŀ�������������SO2�������NaOH��Һ�У�ʹ֮����ȫ����
(6)2.5n
(1)A�Թ��з�������Cu��Ũ��������SO2�ķ�Ӧ��Cu��2H2SO4(Ũ)  CuSO4��SO2����2H2O��(2)����SO2��ʹƷ����Һ��ɫ��������֤��Cu��Ũ���ᷴӦ������SO2���塣(3)��ˮ�е�Cl2���������ԣ��ܽ�SO2����ΪSO42-��SO42-��Ba2����Ӧ����BaSO4��������ˮ���м��ԣ�����SO2����SO32-��SO32-��Ba2����Ӧ����BaSO3������(4)����E�������ͨ����ʹװ���ڵ�ѹǿ�㶨����ֹ���ֵ�������(5)�������ж�����ΪSO2��ֻҪ���ÿ�����SO2��������NaOH��Һ�У�ʹSO2����ȫ���ռ��ɡ�(6)SO2��Na2S��Ӧ��������SO2��H2O��Ӧ����H2SO3��SO2��H2O??H2SO3��H2SO3��Na2S��Ӧ����H2S��Na2S��H2SO3=H2S����Na2SO3��SO2��H2S��Ӧ����S��2H2S��SO2=3S����2H2O���ù��̿ɱ�ʾΪ3SO2��2Na2S=3S����2Na2SO3��n mol Na2S����1.5n mol SO2��ͬʱ����n mol Na2SO3��Na2SO3����SO2����NaHSO3��Na2SO3��SO2��H2O=2NaHSO3���ò���Ӧ�ֿ�����n mol SO2�������������2.5n mol SO2��
CuSO4��SO2����2H2O��(2)����SO2��ʹƷ����Һ��ɫ��������֤��Cu��Ũ���ᷴӦ������SO2���塣(3)��ˮ�е�Cl2���������ԣ��ܽ�SO2����ΪSO42-��SO42-��Ba2����Ӧ����BaSO4��������ˮ���м��ԣ�����SO2����SO32-��SO32-��Ba2����Ӧ����BaSO3������(4)����E�������ͨ����ʹװ���ڵ�ѹǿ�㶨����ֹ���ֵ�������(5)�������ж�����ΪSO2��ֻҪ���ÿ�����SO2��������NaOH��Һ�У�ʹSO2����ȫ���ռ��ɡ�(6)SO2��Na2S��Ӧ��������SO2��H2O��Ӧ����H2SO3��SO2��H2O??H2SO3��H2SO3��Na2S��Ӧ����H2S��Na2S��H2SO3=H2S����Na2SO3��SO2��H2S��Ӧ����S��2H2S��SO2=3S����2H2O���ù��̿ɱ�ʾΪ3SO2��2Na2S=3S����2Na2SO3��n mol Na2S����1.5n mol SO2��ͬʱ����n mol Na2SO3��Na2SO3����SO2����NaHSO3��Na2SO3��SO2��H2O=2NaHSO3���ò���Ӧ�ֿ�����n mol SO2�������������2.5n mol SO2��
 CuSO4��SO2����2H2O��(2)����SO2��ʹƷ����Һ��ɫ��������֤��Cu��Ũ���ᷴӦ������SO2���塣(3)��ˮ�е�Cl2���������ԣ��ܽ�SO2����ΪSO42-��SO42-��Ba2����Ӧ����BaSO4��������ˮ���м��ԣ�����SO2����SO32-��SO32-��Ba2����Ӧ����BaSO3������(4)����E�������ͨ����ʹװ���ڵ�ѹǿ�㶨����ֹ���ֵ�������(5)�������ж�����ΪSO2��ֻҪ���ÿ�����SO2��������NaOH��Һ�У�ʹSO2����ȫ���ռ��ɡ�(6)SO2��Na2S��Ӧ��������SO2��H2O��Ӧ����H2SO3��SO2��H2O??H2SO3��H2SO3��Na2S��Ӧ����H2S��Na2S��H2SO3=H2S����Na2SO3��SO2��H2S��Ӧ����S��2H2S��SO2=3S����2H2O���ù��̿ɱ�ʾΪ3SO2��2Na2S=3S����2Na2SO3��n mol Na2S����1.5n mol SO2��ͬʱ����n mol Na2SO3��Na2SO3����SO2����NaHSO3��Na2SO3��SO2��H2O=2NaHSO3���ò���Ӧ�ֿ�����n mol SO2�������������2.5n mol SO2��
CuSO4��SO2����2H2O��(2)����SO2��ʹƷ����Һ��ɫ��������֤��Cu��Ũ���ᷴӦ������SO2���塣(3)��ˮ�е�Cl2���������ԣ��ܽ�SO2����ΪSO42-��SO42-��Ba2����Ӧ����BaSO4��������ˮ���м��ԣ�����SO2����SO32-��SO32-��Ba2����Ӧ����BaSO3������(4)����E�������ͨ����ʹװ���ڵ�ѹǿ�㶨����ֹ���ֵ�������(5)�������ж�����ΪSO2��ֻҪ���ÿ�����SO2��������NaOH��Һ�У�ʹSO2����ȫ���ռ��ɡ�(6)SO2��Na2S��Ӧ��������SO2��H2O��Ӧ����H2SO3��SO2��H2O??H2SO3��H2SO3��Na2S��Ӧ����H2S��Na2S��H2SO3=H2S����Na2SO3��SO2��H2S��Ӧ����S��2H2S��SO2=3S����2H2O���ù��̿ɱ�ʾΪ3SO2��2Na2S=3S����2Na2SO3��n mol Na2S����1.5n mol SO2��ͬʱ����n mol Na2SO3��Na2SO3����SO2����NaHSO3��Na2SO3��SO2��H2O=2NaHSO3���ò���Ӧ�ֿ�����n mol SO2�������������2.5n mol SO2��
��ϰ��ϵ�д�
�����Ŀ

 Fe2O3��SO2����SO3�����������ɵ�����ͨ���Ȼ�����Һ�У��õ��ij�������( )
Fe2O3��SO2����SO3�����������ɵ�����ͨ���Ȼ�����Һ�У��õ��ij�������( ) �� 60mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
�� 60mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�