题目内容
11.下列实验操作中,说法正确的是( )| A. | 燃料电池的制作:用包有薄海绵的两根碳棒做电极电解Na2SO4溶液,一段时间后切断电源,在两极之间接上发光二极管,发现二极管发光 | |
| B. | 滴定管、移液管以及滴定过程中用于盛待测液的锥形瓶,使用前都需要洗涤与润洗 | |
| C. | 通常用产生气泡的快慢,比较不同条件下Na2S2O3溶液与稀硫酸反应速率 | |
| D. | 用高锰酸钾溶液滴定Na2SO3溶液至终点:滴入最后一滴高锰酸钾溶液,溶液恰好由紫色变为无色,且半分钟不变色 |
分析 A.电解硫酸钠溶液时一个碳棒上生成氢气、一个碳棒上生成氧气,当切断电源后,该装置构成原电池而产生电流;
B.锥形瓶不能润洗;
C.Na2S2O3+H2SO4=Na2SO4+S↓+SO2↑+H2O,虽说也有SO2产生,但SO2在水中溶解度比较大;
D.高锰酸钾溶液呈紫色,Na2SO3溶液与高锰酸钾溶液恰好反应时,溶液是无色的,当高锰酸钾过量时溶液呈紫色.
解答 解:A.电解硫酸钠溶液时一个碳棒上生成氢气、一个碳棒上生成氧气,当切断电源后,该装置构成原电池而产生电流,导致二极管发光,故A正确;
B.锥形瓶不能润洗,否则易导致测定浓度偏大,故B错误;
C.Na2S2O3+H2SO4=Na2SO4+S↓+SO2↑+H2O,虽说也有SO2产生,但SO2在水中溶解度比较大,1体积水大约可以溶解40体积SO2,观察气体产生快慢不如观察沉淀生成现象明显,故C错误;
D.高锰酸钾溶液呈紫色,Na2SO3溶液与高锰酸钾溶液恰好反应时,溶液是无色的,当高锰酸钾过量时溶液呈紫色,用高锰酸钾溶液滴定Na2SO3溶液至终点:滴入最后一滴高锰酸钾溶液,溶液恰好由无色变为紫色,且半分钟不变色故D错误;
故选A.
点评 本题考查化学实验方案评价,为高频考点,涉及基本操作、中和滴定、原电池原理等知识点,明确实验原理及基本操作规范性是解本题关键,易错选项是B,注意锥形瓶不能润洗,不用干燥.

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
1.短周期元素A、B、C、D的原子序数依次递增,A与C同主族,B与D同主族,A、C原子的最外层电子数之和等于B原子的次外层电子数,D元素原子最外层电子数为电子层数的2倍,四种元素原子的核电荷数之和为36.则下列叙述正确的是( )
| A. | C元素的单质在B元素的单质中燃烧,产物1mol与足量的水反应转移电子数为NA | |
| B. | 由上述元素形成的化合物只有一种具有漂白性 | |
| C. | B、C、D三种元素形成的化合物的水溶液用惰性电极电解时,溶液pH一定不变 | |
| D. | A、B形成化合物的沸点高于A、C形成化合物的沸点,因为前者分子间形成了氢键 |
2.下列实验操作与预期实验目的或实验结论不一致的是
( )
| 选项 | 实验操作及现象 | 实验目的或结论 |
| A | 用硫酸酸化的H2O2溶液滴入Fe(NO3)2溶液中,溶液变黄色 | 可证明氧化性:H2O2比Fe3+强 |
| B | 在0.1mol/L的NaHCO3溶液中,加2滴酚酞显浅红色,微热,溶液颜色加深 | 验证盐类水解反应是吸热反应 |
| C | 将一定量的NaNO3和KCl的混合液加热并浓缩至有晶体析出,趁热过滤 | 得到NaCl晶体 |
| D | 亚硫酸钡固体加入盐酸,有二氧化硫气体生成 | 盐酸的酸性强于亚硫酸 |
| A. | A | B. | B | C. | C | D. | D |
19.下列有关说法正确的是( )
| A. | Na2CO3和NaOH都能抑制水的电离 | |
| B. | 0.1 mol•L-1Na2CO3溶液加水稀释,CO32-的水解程度增大,溶液pH减小 | |
| C. | 酸碱中和滴定实验中,锥形瓶需用待测液润洗2~3次后,再加入待测液 | |
| D. | 常温下,pH=3的盐酸、醋酸分别用水稀释m倍、n倍后pH相同,则m<n |
6.日常生活中常用到化学知识,下列说法不正确的是( )
| A. | 赤潮、白色污染、绿色食品中的“赤”“白”“绿”均指相关物质的颜色 | |
| B. | 聚氯乙烯塑料有毒,不可用于制食品包装袋 | |
| C. | 明矾溶于水生成的Al (OH)3胶体,可除去水中的悬浮颗粒等杂质 | |
| D. | 新制的Cu(OH)2可以测定糖尿病患者尿中萄葡糖的含量 |
16.化学与生活、生产息息相关,下列说法中错误的是( )
| A. | 海鲜中有较多Zn元素,经常食用可提高免疫力 | |
| B. | 聚乳酸可用作手术缝合纱线、缓释药物材料 | |
| C. | 氨冷冻系统可用于禽类加工厂的冷库 | |
| D. | 用浓硫酸处理碱性工业废水 |
3.下列关于有机化合物的说法正确的是( )
| A. | 乙酸和乙酸乙酯可用Na2CO3溶液加以区别 | |
| B. | 乙烯和苯都能使溴水褪色,褪色的原因相同 | |
| C. | 蛋白质和油脂都属于高分子化合物,一定条件下能水解 | |
| D. | 煤油可由煤干馏获得,可用作燃料和保存少量金属钠 |
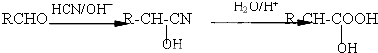
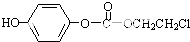 ,该物质在NaOH水溶液中加热反应时的化学方程式为
,该物质在NaOH水溶液中加热反应时的化学方程式为 +5NaOH
+5NaOH +HOCH2CH2OH+Na2CO3+NaCl+2H2O.
+HOCH2CH2OH+Na2CO3+NaCl+2H2O. .
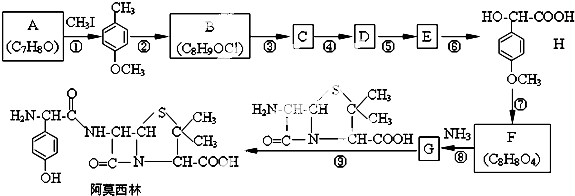
.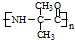 的合成路线流程图(无机试剂任用).
的合成路线流程图(无机试剂任用).
 .
.