��Ŀ����
8����1��ijͬѧ��ʵ����ģ���Ʊ����0.600mol NH3��35.100gʳ�λ�ϣ��õ�����ˮ������Ϊ105.300g�������Һͨ��CO2����Ӧ��ȫ�����ˣ��õ���Һ88.200g����NaHCO3�IJ���Ϊ86.3%����������λС������2��һ�������£�85.5g��������ͳ����Ļ������22.4L��������Ϊ������Ҹû���������ͬ״��������������ܶ�Ϊ20.8��ǡ����ȫ��Ӧ���õ��������ӻ�����A��B�Ĺ������A��B�У��������Ӹ����Ⱦ�Ϊ1��1�Ҿ���������������������A�ĺ�����Ϊ0.3596������A�Ļ�ѧʽΪRbO3��
����������������ȣ�A�ɷֽ�����B������������һ��ʱ��������������������ӵ����ʵ�����ȣ���ֽ�����������ڱ���µ����Ϊ1.12L��
���� ��1�����ݷ�Ӧ����ʽNaCl+CO2+NH3+H2O��NaHCO3��+NH4Cl�ɼ���̼�����Ƶ����۲��������������غ㶨�ɿɼ���ʵ�ʲ���������=$\frac{ʵ�ʲ���}{���۲���}��100%$��
��2�����ݺ����������A�Ļ�ѧʽ�����������غ�������������������������������������
��� �⣺��1��35.1gʳ�ε����ʵ���Ϊ��$\frac{35.1g}{58.5g/mol}$=0.6mol��
NaCl+CO2+NH3+H2O��NaHCO3��+NH4Cl
0.6mol 0.6mol 0.6mol
�����Ͽ�����̼�����Ƶ�����Ϊ��0.6mol��84g/mol=50.4g��
���������غ㶨�ɣ�ʵ�ʵõ�̼�����Ƶ�����Ϊ��105.300g+0.6mol��44g/mol-88.200g=43.500g��
��̼�����ƵIJ���Ϊ��$\frac{43.500g}{50.4g}��100%$=86.3%��
�ʴ�Ϊ��86.3%��
��2��A��墨��������ʵ���֮��Ϊ��$\frac{1-0.3596}{85.5}$��$\frac{0.3596}{16}$=1��3����A�Ļ�ѧʽΪ��RbO3��
A�ɷֽ�����B��������A��B�У��������Ӹ����Ⱦ�Ϊ1��1�Ҿ�����������������������B�Ļ�ѧʽΪ��RbO2������һ��ʱ��������������������ӵ����ʵ�����ȣ���RbO2��RbO3���ʵ�����ȣ���Ϊ0.5mol������������Ϊ��0.5mol��32g/mol+0.5mol��48g/mol=40g��
��������ƽ��Ħ������Ϊ��20.8��2g/mol=41.6g/mol������£�22.4L�����������ʵ���Ϊ1mol������Ϊ41.6g����A�ֽ������������Ϊ41.6g-40g=1.6g�����ʵ���Ϊ��$\frac{1.6g}{32g/mol}$=0.05mol����������Ϊ��0.05mol��22.4L/mol=1.12L��
�ʴ�Ϊ��RbO3��1.12L��
���� ���⿼���˸��ݷ���ʽ�ļ��㣬�е��Ѷȣ�ע�������غ㶨�ɵ�Ӧ�ã���A�Ļ�ѧʽ�������Ϣ�Ƴ�B�Ļ�ѧʽ�ǽ���ؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����ʹ�õ�ʯ�͡�ú�ȴ�ͳ��Դ���������ܡ����ܵ�����Դ����������Щ��Դ������̫����ת���� | |
| B�� | ��������һ�����ܵ������ﱡĤ���м�ǿ�Ŀ���ʴ������������������������������ԡ�������ʳƷ�� | |
| C�� | �������ò�ľ�������ɻ�����ũҵ���ø������ֽ��֣���ҵ�����������δ���Ƥ��ȣ�����̵Ĺ�ͬ�㶼�������˵����ʱ��Ե����� | |
| D�� | �������������Ρ��������ȵȺ��Ȼ����ﶼ����ǿ�����ԣ�������ɱ���������Ȼ��ء������Ҳ�Ǻ��Ȼ������Ҳ������ɱ������ |
| A�� | ��ˮ���������c��H+ ��=l.0xl0-12 mol/L | |
| B�� | c ��CH3COOH����c ��H+ ����c ��CH3COO- ����c ��OH-�� | |
| C�� | ��ͬŨ�ȵ�����ֱ��ˮϡ��10����pH �����ᣩ��pH �����ᣩ | |
| D�� | ������������������Һ��Ӧ��c ��CH3COOH��+c ��CH3COO- ��=0.01mol/L�� |
| A�� | ���������ʱ�����ɵ��������������ӵ����� | |
| B�� | ���������ʱ�����ɵ������������������ӵ��Ǽ� | |
| C�� | ���������ʱ�����ɽ��������Ӻ�������ӵ����� | |
| D�� | NH4Cl���� |
| A�� | MgO+Al | B�� | ������ڵ�MgCl2 | C�� | ����MgCl2 | D�� | MgO+C�����£� |
 [���ʽṹ������]���������з�Ӧ�ϳ������谷��
[���ʽṹ������]���������з�Ӧ�ϳ������谷��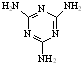 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ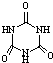 �������������������谷�����֮��ͨ�����Ӽ������ϣ������������γɽ�ʯ��
�������������������谷�����֮��ͨ�����Ӽ������ϣ������������γɽ�ʯ��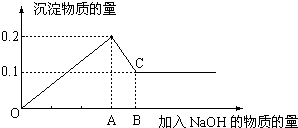
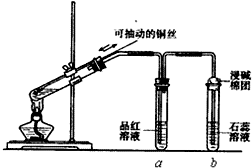 ijͬѧ���ʵ��֤��ͭ��Ũ�����ܷ�����Ӧ��������������������ʣ���ͼ��ʾ�����Թ������2mLŨ���ᣬ�ô����ܺ�һ��С�Ľ����������ӿ��в���һ��ͭ˿�����ȣ��ѷų�����������ͨ��Ʒ����Һ��ʯ����Һ�У�
ijͬѧ���ʵ��֤��ͭ��Ũ�����ܷ�����Ӧ��������������������ʣ���ͼ��ʾ�����Թ������2mLŨ���ᣬ�ô����ܺ�һ��С�Ľ����������ӿ��в���һ��ͭ˿�����ȣ��ѷų�����������ͨ��Ʒ����Һ��ʯ����Һ�У�