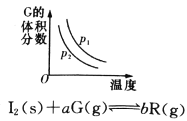
题目内容
【题目】已知:(1)H2(g) +1/2O2(g)=H2O(g) △H=-241.8 kJ/mol
(2)1/2N2 (g) + O2(g)=NO2(g) △H=+33.9 kJ/mol
(3)1/2N2 (g) +3/2 H2(g)=NH3(g) △H=-46.0 kJ/mol
计算NH3(g)燃烧生成NO2(g)和H2O(g)的燃烧热
A.282.8 kJ/mol B.-282.8 kJ/mol C.848.4kJ/mol D.-848.4 kJ/mol
【答案】A
【解析】
试题分析:①H2(g)+![]() O2(g)=H2O(g)△H1=-241.8kJmol-1,②
O2(g)=H2O(g)△H1=-241.8kJmol-1,②![]() N2(g)+O2(g)=NO2(g)△H2=+33.9kJmol-1,③
N2(g)+O2(g)=NO2(g)△H2=+33.9kJmol-1,③![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g)△H3=-46.0kJmol-1,依据热化学方程式和盖斯定律计算①×
H2(g)=NH3(g)△H3=-46.0kJmol-1,依据热化学方程式和盖斯定律计算①×![]() +②-③得到NH3(g)燃烧生成NO2 (g)和H2O(g) 热化学方程式:NH3(g)+
+②-③得到NH3(g)燃烧生成NO2 (g)和H2O(g) 热化学方程式:NH3(g)+![]() O2(g)=NO2(g)+
O2(g)=NO2(g)+![]() H2O(g)△H=-282.8kJmol-1 ,故选A。
H2O(g)△H=-282.8kJmol-1 ,故选A。

 阅读快车系列答案
阅读快车系列答案【题目】
产品标准 | GB5461 |
产品等级 | 一级 |
配 料 | 食盐、碘酸钾、抗结剂 |
碘含量(以I计) | 20~50mg/kg |
(1)碘酸钾与碘化钾在酸性条件下发生如下反应,配平化学方程式(将化学计量数填于空白处)
KIO3+ KI+ H2SO4= K2SO4+ I2+ H2O
(2)上述反应生成的I2可用四氯化碳检验。向碘的四氯化碳溶液中加入Na2SO3稀溶液,将I2还原,以回收四氯化碳。
①Na2SO3稀溶液与I2反应的离子方程式是 .
②某学生设计回收四氯化碳的操作为:
a.将碘的四氯化碳溶液置于分液漏斗中;
b.加入适量Na2SO3稀溶液;
c.分离出下层液体;
d.将分液漏斗充分振荡后静置
其中分液漏斗使用前须进行的操作是 ,上述操作正确的顺序是 (填序号)
(3)已知:I2+2S2O32﹣═2I﹣+S4O62﹣.某学生测定食用精制盐的碘含量,其步骤为:
a.准确称取wg食盐,加适量蒸馏水使其完全溶解;
b.用稀硫酸酸化所得溶液,加入足量KI溶液,使KIO3与KI反应完全;
c.以淀粉为指示剂,逐滴加入物质的量浓度为2.0×10﹣3mol· L﹣1的Na2S2O3溶液10.0mL,恰好反应完全。
①判断c中反应恰好完全依据的现象是 。
②b中反应所产生的I2的物质的量是 mol。
③根据以上实验和包装袋说明,所测精制盐的碘含量是(以含w的代数式表示) mg/kg。