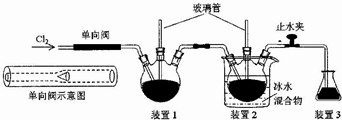
��Ŀ����
2��ijͬѧ�ڳ������������ʵ������̽��Na2S2O3�Ļ�ѧ���ʣ�pH=8$\underset{\stackrel{��}{��}}{pH��ֽ}$Na2S2O3��Һ$��_{ͬʱ�����Ȼ�����Һ}^{�ڼ���������ˮ}$��ɫ����B����˵����ȷ���ǣ�������
| A�� | ʵ���˵��Na2S2O3��Һ��ˮ�����c��OH-��=l0-8 mol��L-l | |
| B�� | Na2S2O3��ҺpH=8��ԭ�������ӷ���ʽ��ʾΪS2O32-+2H2O?Na2S2O3+2OH- | |
| C�� | ���ɵij���B������BaSO3��BaSO4��Ҫ��һ��ȷ�ϻ����ټ���ϡ������֤ | |
| D�� | ʵ���˵��Na2S2O3���л�ԭ�� |
���� �����£���pH=8$\underset{\stackrel{��}{��}}{pH��ֽ}$Na2S2O3��Һ��֪��pH=8����Һˮ���Լ��ԣ�c��H+��ˮ=c��OH-��ˮ=l0-6 mol��L-l����Na2S2O3��Һ$��_{ͬʱ�����Ȼ�����Һ}^{�ڼ���������ˮ}$��ɫ����B��֪������S2O32-+5H2O+4Cl2+2Ba2+=2BaSO4��+8Cl-+10H+���Դ������
��� �⣺A��ʵ���˵��Na2S2O3��Һ��ˮ�����c��OH-��=l0-6 mol��L-l���ٽ�ˮ�ĵ��룬��A����
B��Na2S2O3��ҺpH=8��ԭ�������ӷ���ʽ��ʾΪS2O32-+H2O?HS2O3-+OH-����B����
C��BaSO3�����ᷢ��������ԭ��Ӧ����BaSO4�����ܼ�������֤����C����
D��ʵ��ڷ���S2O32-+5H2O+4Cl2+2Ba2+=2BaSO4��+8Cl-+10H+��SԪ�صĻ��ϼ����ߣ�˵��Na2S2O3���л�ԭ�ԣ���D��ȷ��
��ѡD��
���� ���⿼������ʵ�鷽������ƣ�Ϊ��Ƶ���㣬�漰����ˮ�⡢������ԭ��Ӧ�ȣ����շ�Ӧԭ�������ʵ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
11��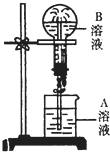 ��ͼΪ��Ȫʵ��װ��ͼ��
��ͼΪ��Ȫʵ��װ��ͼ��
��1��������ƿ�ڳ����Ϊ����NH3��Ҫʹ��Ȫ�γɱ��еĸ�����ɫ����д��A��Һ�����ʣ��������й����ӱ�ʾ����д�����ӷ��ţ������ƻ����ӷ��ţ�
��2������ʵ�����B��Һ���ɫ��ԭ��Ϸ���ʽ˵����NH3+H2O NH3•H2O
NH3•H2O NH4++OH-����Һ�ʼ��ԣ�����̪�Ժ�ɫ��ʵ��ڷ�Ӧ�����ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��
NH4++OH-����Һ�ʼ��ԣ�����̪�Ժ�ɫ��ʵ��ڷ�Ӧ�����ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��
 ��ͼΪ��Ȫʵ��װ��ͼ��
��ͼΪ��Ȫʵ��װ��ͼ����1��������ƿ�ڳ����Ϊ����NH3��Ҫʹ��Ȫ�γɱ��еĸ�����ɫ����д��A��Һ�����ʣ��������й����ӱ�ʾ����д�����ӷ��ţ������ƻ����ӷ��ţ�
| ʵ���� | A��Һ��ɫ | B��Һ��ɫ | A��Һ������ |
| �� | ��ɫ | ��ɫ | ��̪ |
| �� | ��ɫ | ���ɫ���� | Fe3+��FeCl3�ȣ� |
| �� | ��ɫ | ��ɫ | ʯ�� |
| �� | ��ɫ | ���ɫ���� | Al3+��Mg2+��AlCl3��MgCl2�ȣ� |
 NH3•H2O
NH3•H2O NH4++OH-����Һ�ʼ��ԣ�����̪�Ժ�ɫ��ʵ��ڷ�Ӧ�����ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��
NH4++OH-����Һ�ʼ��ԣ�����̪�Ժ�ɫ��ʵ��ڷ�Ӧ�����ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��
10���������ʵ���������ȷ���ǣ�������
| A�� | ����CCl4��ȡ����еĵ� | |
| B�� | ����ɫ��Ӧʵ��ʱ������ɫ�ܲ������۲쵽������ɫΪ��ɫ��֤����������Ϊһ��Ϊ���� | |
| C�� | ������Ũ����Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ��2%��5%��������Һ | |
| D�� | �����£�Ũ��Ϊ0.1mol•L-1Na2S2O3��H2SO4��Һ���ֱ�ȡ5mL��10mL��ϣ�10mL��10mL��ϣ�����֤Na2S2O3Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
7������������ȷ���ǣ�������
| A�� | ����Դ�������ƹ���ʹ�������ڼ��ٹ⻯ѧ�����IJ��� | |
| B�� | Ϊ�˷�ֹ�±��ȸ�֬ʳƷ�������ʣ����ڰ�װ���з�����ʯ�� | |
| C�� | ��̼������ĭ������Ϊ������̬�ĵ���̼������ʯī����Ϊͬλ�� | |
| D�� | ��ˮ�����ܽ����ˮ��ӦΣ������ˮ�м��뾻ˮ����������ʹ��ˮ���� |
14��һ�������£�������ͼ��ʾװ�ÿ�ʵ���л���Ĵ��⣬�����й�˵����ȷ���ǣ�������


| A�� | ���·���ӵ��ƶ�����A����Դ��B | |
| B�� | ����X�ڷ�Ӧ��ͨ�����ֻ�ԭ�� | |
| C�� | �缫DΪ���Ե缫��EΪ���õ缫 | |
| D�� | �缫D�ĵ缫��ӦʽΪC6H6+6H++6e-�TC6H12 |
11�����еķ�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A�� | ��������Һ�м������[Ag��NH3��2]++OH-+3H+=Ag++2NH4++H2O | |
| B�� | ������Mg��OH��2����Һ�м����Ȼ��Ũ��Һ��Mg��OH��2+2NH4+=2NH3•H2O+Mg2+ | |
| C�� | ����������Һ�м���������Һ��2Fe3++3S2-+6H2O=2Fe��OH��3��+3H2S�� | |
| D�� | NaHCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3-+OH-+Ba2+=H2O+BaCO3�� |
12�����Ե���������ŵ��������ص㣬���㷺Ӧ�ã�п-�̼��Ե��������������ҺΪ���Һ������ܷ�ӦʽΪ��Zn+2MnO2+H2O=Zn��OH��2+Mn2O3������˵��������ǣ�������
| A�� | ��ع���ʱ��пʧȥ���� | |
| B�� | ��������ĵ缫��ӦʽΪ2MnO2+H2O+2e-�TMn2O3+2OH- | |
| C�� | ���·��ÿͨ��0.2mol���ӣ�п�����������ϼ���13g | |
| D�� | ��ع���ʱ�������ɸ���ͨ�����·�������� |