��Ŀ����
12�����Ե���������ŵ��������ص㣬���㷺Ӧ�ã�п-�̼��Ե��������������ҺΪ���Һ������ܷ�ӦʽΪ��Zn+2MnO2+H2O=Zn��OH��2+Mn2O3������˵��������ǣ�������| A�� | ��ع���ʱ��пʧȥ���� | |
| B�� | ��������ĵ缫��ӦʽΪ2MnO2+H2O+2e-�TMn2O3+2OH- | |
| C�� | ���·��ÿͨ��0.2mol���ӣ�п�����������ϼ���13g | |
| D�� | ��ع���ʱ�������ɸ���ͨ�����·�������� |
���� ���ݵ�ط�Ӧʽ֪��Zn�ڷ�Ӧ�л��ϼ���0�۱�Ϊ+2�ۣ�����Zn�������������������������������Һ�ʼ��ԣ�������ӦʽΪZn-2e-+2OH-�TZn��OH��2��������ӦʽΪ2MnO2+H2O+2e-=Mn2O3+2OH-�����ӴӸ����ص��������������ݴ˷������
��� �⣺A��Zn�ڷ�Ӧ�л��ϼ���0�۱�Ϊ+2�ۣ����Էŵ�ʱпʧ���ӷ���������Ӧ����A��ȷ��
B�������ϵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2MnO2+H2O+2e-�TMn2O3+2OH-����B��ȷ��
C�����·��ÿͨ��0.2mol���ӣ�����Zn-2e-+2OH-�TZn��OH��2֪������п������=$\frac{0.2mol}{2}��65g/mol$=6.5g����C����
D���ŵ�ʱ��������ʧ���ӡ������ϵõ��ӣ����Ե��ӴӸ����ص���������������D��ȷ��
��ѡC��
���� ���⿼�黯ѧ��Դ���͵�أ�Ϊ�߿���Ƶ�㣬��ȷ��д�缫��Ӧʽ�ǽⱾ��ؼ���Ҳ���ѵ㣬Ҫ��ϵ������Һ�������д��֪�����ӡ��������������Һ�����������ƶ�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
2��ijͬѧ�ڳ������������ʵ������̽��Na2S2O3�Ļ�ѧ���ʣ�pH=8$\underset{\stackrel{��}{��}}{pH��ֽ}$Na2S2O3��Һ$��_{ͬʱ�����Ȼ�����Һ}^{�ڼ���������ˮ}$��ɫ����B
����˵����ȷ���ǣ�������
����˵����ȷ���ǣ�������
| A�� | ʵ���˵��Na2S2O3��Һ��ˮ�����c��OH-��=l0-8 mol��L-l | |
| B�� | Na2S2O3��ҺpH=8��ԭ�������ӷ���ʽ��ʾΪS2O32-+2H2O?Na2S2O3+2OH- | |
| C�� | ���ɵij���B������BaSO3��BaSO4��Ҫ��һ��ȷ�ϻ����ټ���ϡ������֤ | |
| D�� | ʵ���˵��Na2S2O3���л�ԭ�� |
20��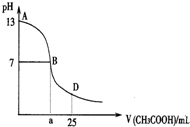 ��ͼΪ��25mL 0.1mol•L-1 NaOH��Һ����εμ�0.2mol•L-1CH3COOH��Һ��������ҺpH�ı仯���ߣ�AB���䣬c��OH-����c��H+������c��OH-����
��ͼΪ��25mL 0.1mol•L-1 NaOH��Һ����εμ�0.2mol•L-1CH3COOH��Һ��������ҺpH�ı仯���ߣ�AB���䣬c��OH-����c��H+������c��OH-����
c��CH3COO-����С��ϵ�ǣ�������
 ��ͼΪ��25mL 0.1mol•L-1 NaOH��Һ����εμ�0.2mol•L-1CH3COOH��Һ��������ҺpH�ı仯���ߣ�AB���䣬c��OH-����c��H+������c��OH-����
��ͼΪ��25mL 0.1mol•L-1 NaOH��Һ����εμ�0.2mol•L-1CH3COOH��Һ��������ҺpH�ı仯���ߣ�AB���䣬c��OH-����c��H+������c��OH-����c��CH3COO-����С��ϵ�ǣ�������
| A�� | c��OH-��һ������c��CH3COO-�� | B�� | c��OH-��һ��С��c��CH3COO-�� | ||
| C�� | c��OH-��һ������c��CH3COO-�� | D�� | c��OH-�����ڡ�С�ڻ����c��CH3COO-�� |
17����֪A��B��C��D��E�Ƕ�������ԭ���������������5������Ԫ�أ�����Ԫ��A��E�ĵ����ڳ����³���̬��Ԫ��B��ԭ������������������Ӳ�����2����Ԫ��C��ͬ���ڵ�����Ԫ����ԭ�Ӱ뾶���Ԫ��D�ĺϽ����ճ������г��õĽ������ϣ�����˵����ȷ���ǣ�������
| A�� | Ԫ��A��B��ɵĻ����ﳣ����һ������̬ | |
| B�� | ����������Ӧˮ����ļ��ԣ�C��D | |
| C�� | ������AE��CE���в�ͬ���͵Ļ�ѧ�� | |
| D�� | һ�������£�Ԫ��C��D������������Ӧ��ˮ����֮�䲻�ܷ�����Ӧ |
1������˵������ȷ���ǣ�������
| A�� | ����ʯ��ˮ�м���һ������ʯ�ң��¶��������ߣ�������Һ��pH���� | |
| B�� | AgCl����Һ�д���ƽ�⣺AgCl��s���TAg+��aq��+Cl-��aq���������м�������NaCl��ĩ��ƽ�������ƶ�����Һ�����ӵ���Ũ�Ȼ��С | |
| C�� | AgCl����Һ�м���KI��Һ����ɫ������ɻ�ɫ��֤����������Ksp��AgCl����Ksp��AgI�� | |
| D�� | Ӳˮ�к��н϶��Ca2+?Mg2+?HCO3-��SO42-��������п�����ȫ��ȥ���е�Ca2+?Mg2+ |
2������ͼʾ��ʵ���������ʵ����Ӧʵ��Ŀ���ǣ�������
| A�� |  ֤��װ�õ����������� | |
| B�� | 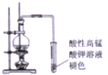 ֤���Ҵ���ŨH2SO4���������˴�����ϩ | |
| C�� |  ֤��SO2�л�ԭ�� | |
| D�� |  �ñ���ʳ��ˮ��CaC2�Ʊ����ռ�C2H2 |
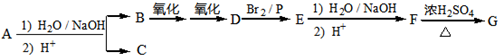

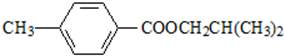 ��
�� ���÷�Ӧ����ȡ����Ӧ����������Ӧ�����Ӧ���ͣ���
���÷�Ӧ����ȡ����Ӧ����������Ӧ�����Ӧ���ͣ���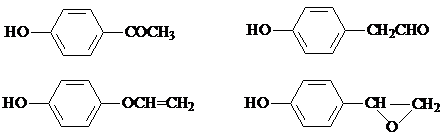 ��
��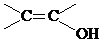 ���ṹ��
���ṹ��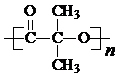 ��
��