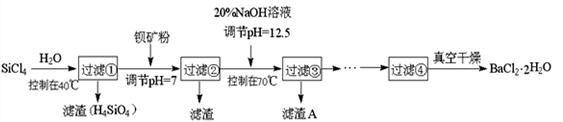
��Ŀ����
��������dz��õ�����������ҵ�������̿���Ҫ�ɷ���MnO2��Ϊԭ���Ʊ�������ؾ��塣��ͼ��ʵ�����Ʊ��IJ������̣�
������Ӧ�ڵĻ�ѧ����ʽ��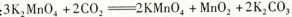
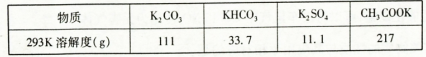
��֪��
��1���������̿�KClO3��KOH����ʱ�������ô�������ѡ����������������_______��
��Ӧ�ٵĻ�ѧ����ʽΪ______��
��2������Һ�еõ�KMnO4�����ʵ�����������________��ѡ����ĸ���ţ���ͬ����
A������ B������ C������ D������ E����ȴ�ᾧ
��3���Ʊ���������Ҫ�õ�������CO2���塣��ȡ��CO2�����ѡ�������Լ���_________��
A��ʯ��ʯ B��Ũ���� C��ϡ���� D������
��4��ʵ��ʱ����CO2����������KHCO3�����µõ���KMnO4��Ʒ�Ĵ��Ƚ��͡�ԭ����______ ��
��5������CO2��ͨ�������ѿ��ƣ���˶�����ʵ�鷽�������˸Ľ�������ʵ����ͨCO2��Ϊ���������ᡣ�������Ϸ�����ѡ����������________ ���õ��IJ�Ʒ���ȸ��ߡ�
A������ B��Ũ���� C��ϡ����
��1��������ǿ��Ҫ��ʴ����������������Ҫ�ɷ��ǹ����Σ����ж������裬Ҫ��Ӧ����3MnO2��KClO3��6KOH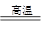 3K2MnO4��KCl��3H2O��2��B E D ��3��C D��4��KHCO3���ܽ�ȱ�K2CO3С�ܶ࣬��Һ����Ũ��ʱKHCO3����KMnO4һ��ᾧ��������5��A����Ϊ�����л�ԭ�ԣ�����ص��ܽ�ȱ�����ش�ܶ࣬����Ũ��ʱ�����϶�������ĸҺ�
3K2MnO4��KCl��3H2O��2��B E D ��3��C D��4��KHCO3���ܽ�ȱ�K2CO3С�ܶ࣬��Һ����Ũ��ʱKHCO3����KMnO4һ��ᾧ��������5��A����Ϊ�����л�ԭ�ԣ�����ص��ܽ�ȱ�����ش�ܶ࣬����Ũ��ʱ�����϶�������ĸҺ�
���������������1�� �������̿�KClO3��KOH��������ʱ������KOH����������е�SiO2������Ӧ��2KOH+SiO2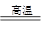 K2SiO3+H2O��ʴ������������Fe������Ӧ������Ҫ�������������������֪��Ӧ�ٵĻ�ѧ����ʽΪ3MnO2��KClO3��6KOH
K2SiO3+H2O��ʴ������������Fe������Ӧ������Ҫ�������������������֪��Ӧ�ٵĻ�ѧ����ʽΪ3MnO2��KClO3��6KOH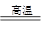 3K2MnO4��KCl��3H2O �� ��2������Һ�еõ�KMnO4�����ʵ���������������Ũ������ȴ�ᾧ�����ˡ����ѡ��ΪB��E��D����3���Ʊ���������Ҫ�õ�������CO2���壬��Ӧ���ò��ӷ��Ե��ᡪ��H2SO4������H2SO4��CaCO3������Ӧ����CaSO4����ˮ��������CaCO3�ı��棬�Ƿ�Ӧ�����ٽ��С�������Ӧ����Na2CO3.ѡ��ΪC��D����4��ʵ��ʱ����CO2����������KHCO3�����µõ���KMnO4��Ʒ�Ĵ��Ƚ��͡�ԭ����KHCO3���ܽ�ȱ�K2CO3С�ܶ࣬��Һ����Ũ��ʱKHCO3����KMnO4һ��ᾧ��������5�� ����ʵ����ͨCO2��Ϊ���������ᣬ��Ϊ�лӷ��ԣ�����ѡ�ã�K2SO4���ܽ�ȱ�CH3COOKС�Ķ࣬����ۺϿ���Ӧ��ѡ�����õ��IJ�Ʒ���ȸ��ߡ���ѡ��ΪA��
3K2MnO4��KCl��3H2O �� ��2������Һ�еõ�KMnO4�����ʵ���������������Ũ������ȴ�ᾧ�����ˡ����ѡ��ΪB��E��D����3���Ʊ���������Ҫ�õ�������CO2���壬��Ӧ���ò��ӷ��Ե��ᡪ��H2SO4������H2SO4��CaCO3������Ӧ����CaSO4����ˮ��������CaCO3�ı��棬�Ƿ�Ӧ�����ٽ��С�������Ӧ����Na2CO3.ѡ��ΪC��D����4��ʵ��ʱ����CO2����������KHCO3�����µõ���KMnO4��Ʒ�Ĵ��Ƚ��͡�ԭ����KHCO3���ܽ�ȱ�K2CO3С�ܶ࣬��Һ����Ũ��ʱKHCO3����KMnO4һ��ᾧ��������5�� ����ʵ����ͨCO2��Ϊ���������ᣬ��Ϊ�лӷ��ԣ�����ѡ�ã�K2SO4���ܽ�ȱ�CH3COOKС�Ķ࣬����ۺϿ���Ӧ��ѡ�����õ��IJ�Ʒ���ȸ��ߡ���ѡ��ΪA��
���㣺���������صĹ�ҵ�Ʒ�����Ҫ�漰�������Լ���ѡ��ѧ����ʽ����д��֪ʶ��

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�CoCl2��6H2O��һ������Ӫ��ǿ������һ������ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��Co(OH)3����������Fe2O3��Al2O3��MnO��)��ȡCoCl2��6H2O�Ĺ����������£�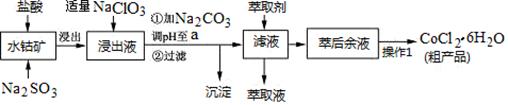
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���(��������Ũ��Ϊ��0.01mol/L)
| ������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��CoCl2��6H2O�۵�Ϊ86�棬������110~120��ʱ��ʧȥ�ᾧˮ������ˮ�Ȼ��ܡ�
��1��д������������Co2O3������Ӧ�����ӷ���ʽ________________________��
��2��д��NaClO3������Ӧ����Ҫ���ӷ���ʽ_____________________________������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽ_______________��
��3������Na2CO3��pH��a��,�������õ��ij����ɷ�Ϊ ��
��4��������1���а���3������ʵ�����������������_________��__________���ˡ��Ƶõ�CoCl2��6H2O�ں��ʱ���ѹ��ɵ�ԭ����__________________��
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ������Һ���м�����ȡ����Ŀ����_________����ʹ�õ����pH��Χ��________________��

A��2.0~2.5 B��3.0~3.5 C��4.0~4.5 D��5.0~5.5
��6��Ϊ�ⶨ�ֲ�Ʒ��CoCl2��6H2O��������ȡһ�������Ĵֲ�Ʒ����ˮ����������AgNO3��Һ�����ˡ�ϴ�ӣ���������ɺ����������ͨ�����㷢�ֲִ�Ʒ��CoCl2��6H2O��������������100������ԭ�������_____________________������һ�����ɣ�
ijѧϰС����ģ���ij������Һ�л��ձ�ͪ���Ҵ��������ʵ�顣�ƶ��������������̡�
��֪�÷�Һ����Ҫ�����Ҵ������л����б�ͪ������������������Ҹ��ֳɷֵķе����±���
| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е㣨�棩 | 56.2 | 77.06 | 78 | 117.9 |
��1�����3�ijɷ�Ϊ____________��
��2�����������е���pH��10��Ŀ����________________________________________________________________________________________________________________________________________________��
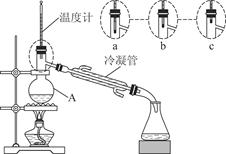
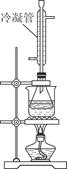
��3����С��ͬѧ������װ����ͼ��ʾ����A���¶ȼƵ�λ����ȷ����________���a����b����c������
��4�����ұ��涨�����ʸ߶�Ũ���Ͱ���������������ƣ�Ӧ������0.30 g/L���������������������ƣ�Ӧ������2.0 g/L��
��Ϊ�ⶨij����Ʒ����������ȡ20.00 mL��Ʒ����ƿ�У������ָ̪ʾ��2�Σ���0.010 mol/L��NaOH����Һ�ζ����յ㡣�ж��յ��������________________________________________________________________________________________________________________________________________________��
���ð���ƷΪ���ʼ���������NaOH��Һ���Ӧ��С��________mL��
�ڰ��е����������÷��η��ⶨ��������ζ������Һ��ǡ�����յ㣩���ټ���20.00mL0.100mol/L NaOH����Һ����ͼװ��ˮԡ���Ȱ�Сʱ����ȴ����0.100mol/L���������Һ�ζ����յ㡣���Ȱ�Сʱ��Ŀ����______________________�������ܵ�������______________����֪���������������Һ7.70 mL���ð���Ʒ��������Ϊ________g/L������С�������λ���֣���
��5�����в�����ʹ�������ⶨ���ƫ�ߵ���________��ѡ���ţ�
a������ʱδʹ��ˮԡ��������
b���ζ�ǰ�ζ����������ݣ��ζ����������
c���ζ���δ���������Һ��ϴ
��(Sr)������������Ԫ�أ��䵥�ʺͻ�����Ļ�ѧ������ơ��������ơ�ʵ�����ú�̼���ȵķ���(��SrCO3 38.40%��SrO12.62%��CaCO3 38.27%��BaCO3 2.54%���������������������8.17%)�Ʊ������ȴ�Ʒ�IJ���ʵ��������£�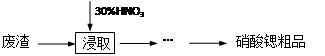
��1������Ũ�������������Ϊ65%���ܶ�Ϊ1.4g/cm3��Ҫ����30%ϡ����500mL������Ҫ���ĵ������� �������ƹ����в�ʹ����ƽ�������Ҫ����������� ������Ҫʹ�õ������� ��
��֪�����ε��ܽ��(g/100 gˮ)���±�
| �¶�/������ | 0 | 20 | 30 | 45 | 60 | 80 | 100 |
| Sr(NO3)2 | 28.2 | 40.7 | 47 | 47.2 | 48.3 | 49.2 | 50.7 |
| Ca(NO3)2��4H2O | 102 | 129 | 152 | 230 | 300 | 358 | 408 |
��2���ɽ�ȡ��õ��Ļ�����Ʊ������ȴ�Ʒ��ʵ�鲽������Ϊ�����ˡ� �� ��ϴ�ӣ����
��֪��������������л��ܼ�A�С�ʽ����Sr(NO3)2�C212��Ba(NO3)2�C261��Ca(NO3)2�C164
��3���Ƶõ������ȴ�Ʒ�к�����Ca(NO3)2��Ba(NO3)2�����ʡ��ⶨ�����ȴ��ȵ�ʵ�����£���ȡ5.39g��������Ʒ�������������л��ܼ�A�������ˡ�ϴ�ӡ������ʣ�����5.26g�����˹������250 mL����Һ��ȡ��25.00 mL������pHΪ7������ָʾ������Ũ��Ϊ0.107mol/L��̼������Һ�ζ����յ㣬����̼������Һ22.98mL��
�ζ����̵ķ�Ӧ��Sr2����CO32���� SrCO3������Ba2����CO32���� BaCO3��
�ٵζ�ѡ�õ�ָʾ��Ϊ ���ζ��յ�۲쵽������Ϊ ��
�ڸ������ȴ�Ʒ�У������ȵ���������Ϊ ��С���������λ�������ζ�ǰ��Ʒ��Ca(NO3)2û�г��������ⶨ�������ȴ��Ƚ��� (�ƫ�ߡ�����ƫ�͡����䡱)��
�����(LiCoO2)����ӵ����һ��Ӧ�ù㷺�����͵�Դ��ʵ���ҳ������÷Ͼ����������ӵ�ػ�����������ͭ���ܡ��Ԫ�أ�ʵ��������£�
(1)����ݹ����У������ܽ�����ӷ���ʽΪ__________________________
(2)��ҺA�м���������Һ��ʹCoԪ����CoC2O4��2H2O������ʽ���������������Ʊ������ܼ��ܷ۵���Ҫԭ�ϡ��ڿ�����CoC2O4��2H2O���ȷֽ�ʧ�����ݼ��±����벹���������е��ȷֽⷽ��ʽ��
| ��� | �¶ȷ�Χ/�� | �ȷֽⷽ��ʽ | ����ʧ���� |
| �� | 120��220 | | 19.67% |
| �� | 280��310 | | 56.10% |
(3)����Li2CO3ʱ������Һ�����������ǣ���ʵ������ĽǶȸ������ֿ��ܵ�ԭ��_____________________________________________________________
(4)�������õ�FeCl3��Һ������ˮ�����Խ�����ӷ���ʽ�����侻ˮԭ��________________________________________________________
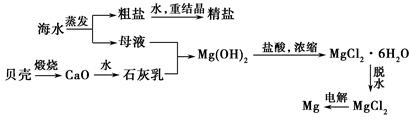
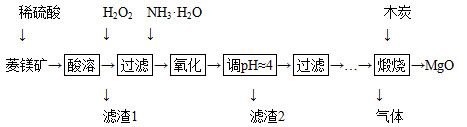
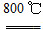 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��