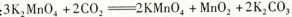��Ŀ����
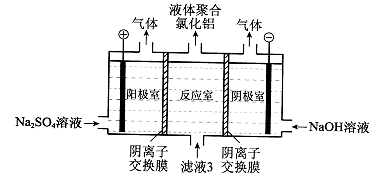
��ˮ���ۺ����ÿ����Ʊ�����þ����������ͼ��ʾ��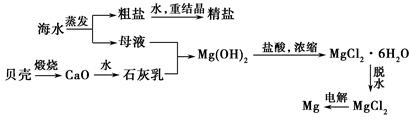
(1)���ڿ����м���MgCl2��6H2O�����ɵ���Mg(OH)Cl��MgO��д����Ӧ��Ӧ�Ļ�ѧ����ʽ�� ��
�õ�ⷨ��ȡ����þʱ����Ҫ��ˮ�Ȼ�þ���ڸ����HCl�����м���MgCl2��6H2Oʱ���ܵõ���ˮMgCl2����ԭ���� ��
(2)Mg(OH)2�����л��е�Ca(OH)2Ӧ������ȥ��д��ʵ�鲽�裺 ��
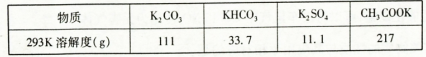
(3)ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ����������ֱ�˵���������������ʹ�ò�������Ŀ�ġ�
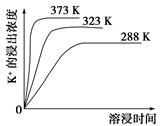
�ܽ�ʱ�� ��
����ʱ�� ��
����ʱ�� ��
(1)MgCl2��6H2O Mg(OH)Cl��HCl����5H2O����MgCl2��6H2O
Mg(OH)Cl��HCl����5H2O����MgCl2��6H2O MgO��2HCl����5H2O����Mg(OH)Cl
MgO��2HCl����5H2O����Mg(OH)Cl MgO��HCl�����ڸ����HCl�����У�������MgCl2ˮ�⣬�Ҵ���MgCl2��6H2O���Ȳ�����ˮ�����ܵõ���ˮMgCl2
MgO��HCl�����ڸ����HCl�����У�������MgCl2ˮ�⣬�Ҵ���MgCl2��6H2O���Ȳ�����ˮ�����ܵõ���ˮMgCl2
(2)����MgCl2��Һ����ֽ��衢���ˣ�����������ˮϴ��
(3)���裬�����ܽ⡡ʹ��Һ�ز���������©������ֹ�������裬��ֹ��ֲ�����Һ�λ���ɽ�
����

���и�����������÷�Һ©���������
| A������ˮ | B�������屽 | C���Ҵ�����ˮ | D��������Ҵ� |
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£�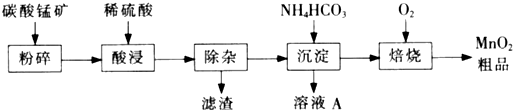 �й��������↑ʼ�����ͳ�����ȫ��pH���±���
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al��OH��2 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
���ʴ��������⣺
��1�����ǰ��̼���̿����������� ��
��2����������Һ�к���Mn2+�� SO42-������������Fe2+��Fe3+��A13+��Cu2+��Pb2+�ȣ�����ӹ������£�
�ټ���MnO2��Fe2+�����������ӷ�Ӧ����ʽΪ �� �ڼ���CaO����Һ��pH����5.2��6.0������ҪĿ���� ��
�ۼ���BaS����ȥCu2+��Pb2+���ټ���NaF��Һ����ȥ ��
��3������ҺA�л��յ���Ҫ������ �������ʳ��������ʡ���4��MnO2��Ʒ�к�������Mn3O4��������ϡ���ᴦ��������ת��ΪMnSO4��MnO2��Ȼ��������������Mn2+ת��ΪMnO2���Ƶ�����MnO2��д��Mn3O4��ϡ���ᷴӦ�Ļ�ѧ����ʽ ��
 2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4 ��2H2O
��2H2O

 ?
? CaCO3(s)��
CaCO3(s)��


 ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬���� ��Һ�����ƽ��ֵΪ19��00mL��
��Һ�����ƽ��ֵΪ19��00mL��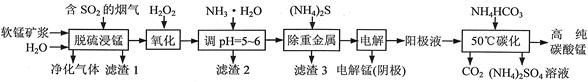
 2Ca2����2K����Mg2����4SO42����2H2O
2Ca2����2K����Mg2����4SO42����2H2O