��Ŀ����
ijѧϰС����ģ���ij������Һ�л��ձ�ͪ���Ҵ��������ʵ�顣�ƶ��������������̡�
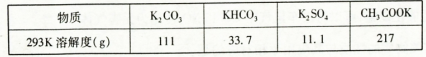
��֪�÷�Һ����Ҫ�����Ҵ������л����б�ͪ������������������Ҹ��ֳɷֵķе����±���
| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е㣨�棩 | 56.2 | 77.06 | 78 | 117.9 |
��1�����3�ijɷ�Ϊ____________��
��2�����������е���pH��10��Ŀ����________________________________________________________________________________________________________________________________________________��
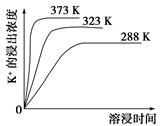
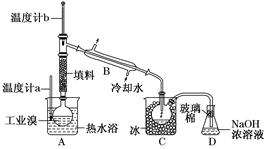
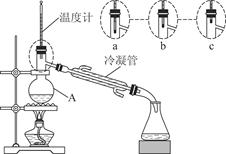
��3����С��ͬѧ������װ����ͼ��ʾ����A���¶ȼƵ�λ����ȷ����________���a����b����c������
��4�����ұ��涨�����ʸ߶�Ũ���Ͱ���������������ƣ�Ӧ������0.30 g/L���������������������ƣ�Ӧ������2.0 g/L��
��Ϊ�ⶨij����Ʒ����������ȡ20.00 mL��Ʒ����ƿ�У������ָ̪ʾ��2�Σ���0.010 mol/L��NaOH����Һ�ζ����յ㡣�ж��յ��������________________________________________________________________________________________________________________________________________________��
���ð���ƷΪ���ʼ���������NaOH��Һ���Ӧ��С��________mL��
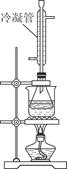
�ڰ��е����������÷��η��ⶨ��������ζ������Һ��ǡ�����յ㣩���ټ���20.00mL0.100mol/L NaOH����Һ����ͼװ��ˮԡ���Ȱ�Сʱ����ȴ����0.100mol/L���������Һ�ζ����յ㡣���Ȱ�Сʱ��Ŀ����______________________�������ܵ�������______________����֪���������������Һ7.70 mL���ð���Ʒ��������Ϊ________g/L������С�������λ���֣���
��5�����в�����ʹ�������ⶨ���ƫ�ߵ���________��ѡ���ţ�
a������ʱδʹ��ˮԡ��������
b���ζ�ǰ�ζ����������ݣ��ζ����������
c���ζ���δ���������Һ��ϴ
��1������
��2��ʹ�������������ƣ�ʹ���������ڼ�������ʱ���������ƺ��Ҵ������ʹ��������ˮ��Ҳ�ɣ�
��3��b
��4������Һ����ɫ��Ϊ�ۺ�ɫ��������ڲ���ɫ��10.00
��ʹ��ˮ����ȫ������������2.024
��5��b
����

 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�������ˮ������ҵ��ɰ�ǡ���֬��Ư����ɱ�������У���������(NaClO2)��������Ҫ�����á���ͼ�������������ƵĹ�������ͼ��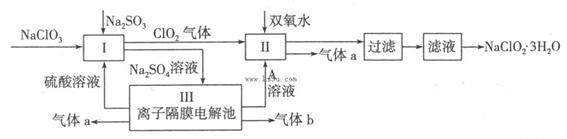
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�ڳ����£�Ksp(FeS)=6��3��10-18��Ksp(CuS)=6��3��10-28��Ksp(PbS)=2��4 ��10-28
��1����ӦI�з�����Ӧ�����ӷ���ʽΪ ��
��2������Һ�еõ�NaClO2��3H2O������������������ (��д���)��
a������ b������Ũ�� c������ d����ȴ�ᾧ e������
��3��ӡȾ��ҵ������������(NaClO2)Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | H2CO3 | H2S |
| Ka��mol��L-1 | 1��10-2 | 6.3��10-4 | K1=4.30��10-7 K2=5.60��10-11 | K1=9.1��10-8 K2=l.1��10-12 |
�ٳ����£����ʵ���Ũ����ȵ�NaClO2��NaF��NaHCO3��Na2S������Һ��pH�ɴ�С��˳��Ϊ (�û�ѧʽ��ʾ)�������ȣ����ʵ���Ũ����ͬ��NaF��NaClO2����Һ�������������������Ĵ�С��ϵΪ�� (�ǰ�ߴ���ȡ����ߴ�)��
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij����� �������£������һ�����ӳ�����ȫʱ(������Ũ��Ϊ10-5mol��L-1)��ʱ��ϵ�е�S2-��Ũ��Ϊ ��
��4����װ������������a�ĵ缫��Ӧʽ ������������a�����Ϊ1��12L(��״��)����ת�Ƶ��ӵ����ʵ���Ϊ ��
ѡ������ʵ�鷽���������ʣ������뷽����������ں����ϡ�
| A����ȡ��Һ | B������ | C���ᾧ | D����Һ E������ F������ G������ |
��2��__ _������غ��Ȼ��ƵĻ��Һ�л������ء�
��3��__ _����ˮ�����͵Ļ���
��4��__ _����CCl4���е�Ϊ76.75�棩�ͼױ����е�Ϊ110.6�棩�Ļ���
 2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4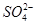 ��2H2O
��2H2O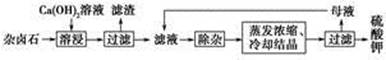

 ?
? CaCO3(s)��
CaCO3(s)�� 2Ca2����2K����Mg2����4SO42����2H2O
2Ca2����2K����Mg2����4SO42����2H2O