��Ŀ����
��(Sr)������������Ԫ�أ��䵥�ʺͻ�����Ļ�ѧ������ơ��������ơ�ʵ�����ú�̼���ȵķ���(��SrCO3 38.40%��SrO12.62%��CaCO3 38.27%��BaCO3 2.54%���������������������8.17%)�Ʊ������ȴ�Ʒ�IJ���ʵ��������£�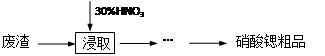
��1������Ũ�������������Ϊ65%���ܶ�Ϊ1.4g/cm3��Ҫ����30%ϡ����500mL������Ҫ���ĵ������� �������ƹ����в�ʹ����ƽ�������Ҫ����������� ������Ҫʹ�õ������� ��
��֪�����ε��ܽ��(g/100 gˮ)���±�
| �¶�/������ | 0 | 20 | 30 | 45 | 60 | 80 | 100 |
| Sr(NO3)2 | 28.2 | 40.7 | 47 | 47.2 | 48.3 | 49.2 | 50.7 |
| Ca(NO3)2��4H2O | 102 | 129 | 152 | 230 | 300 | 358 | 408 |
��2���ɽ�ȡ��õ��Ļ�����Ʊ������ȴ�Ʒ��ʵ�鲽������Ϊ�����ˡ� �� ��ϴ�ӣ����
��֪��������������л��ܼ�A�С�ʽ����Sr(NO3)2�C212��Ba(NO3)2�C261��Ca(NO3)2�C164
��3���Ƶõ������ȴ�Ʒ�к�����Ca(NO3)2��Ba(NO3)2�����ʡ��ⶨ�����ȴ��ȵ�ʵ�����£���ȡ5.39g��������Ʒ�������������л��ܼ�A�������ˡ�ϴ�ӡ������ʣ�����5.26g�����˹������250 mL����Һ��ȡ��25.00 mL������pHΪ7������ָʾ������Ũ��Ϊ0.107mol/L��̼������Һ�ζ����յ㣬����̼������Һ22.98mL��
�ζ����̵ķ�Ӧ��Sr2����CO32���� SrCO3������Ba2����CO32���� BaCO3��
�ٵζ�ѡ�õ�ָʾ��Ϊ ���ζ��յ�۲쵽������Ϊ ��
�ڸ������ȴ�Ʒ�У������ȵ���������Ϊ ��С���������λ�������ζ�ǰ��Ʒ��Ca(NO3)2û�г��������ⶨ�������ȴ��Ƚ��� (�ƫ�ߡ�����ƫ�͡����䡱)��
��1��30%ϡ������ܶ� Ũ���������ˮ����� ��Ͳ���ձ���������
��2�������ᾧ ���ȹ���
��3���ٷ�̪ ��Һ��Ϊ��ɫ��30s����ɫ ��0.95��212x+261y=5.26 x+y=0.0246�� ƫ��
���������������1����������������ȣ�����Ҫ���ĵ�������30%ϡ������ܶ��������ƹ����в�ʹ����ƽ�������Ҫ�����������Ũ���������ˮ�����������Ҫʹ�õ���������Ͳ���ձ�����������
��2���ɱ��е����ݿ��Կ�����Sr(NO3)2���ܽ�������¶ȵ����߱仯��������Ƶ��ܽ�����¶ȱ仯�ϴ���˿�ͨ���ɽ�ȡ��õ��Ļ�����Ʊ������ȴ�Ʒ��ʵ�鲽������Ϊ�������ᾧ�����ȹ��ˣ�ϴ�ӣ�����õ���
��3���ٷ����ζ����̿�֪����Ʒ��Һ��ɫ������̼���Ƴ�����ȫ���Ե����̪��Һָʾ�յ㣬�������һ����Һ�ʺ�ɫ��������ڲ���ɫ���ʴ�Ϊ����̪����Һ���ޱ�Ϊdz��ɫ30���ڲ���ɫ�������ζ�ǰ��Ʒ��Ca��NO3��2û�г����������ı���Һ̼���ƣ����ݵζ���������c������Һ��=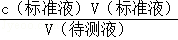 �����ı�Һ�࣬���ⶨ�������ȴ��Ȼ�ƫ�ߣ��ʴ�Ϊ��ƫ�ߡ���֪��������������л��ܼ�A�У�����Ϊ5.39-5.26=0.03g����̼�����Ӧ��ֻ�б����Ӻ������ӣ�Ũ��Ϊ0.107mol/L��̼������Һ22.98mL�����ʵ���Ϊ0.00246mol�������ᱵ�������ȵ����ʵ���Ϊy��x����������ʽΪ212x+261y=5.26 x+y=0.0246�����������ȵ�������������5.39��ȥ����ƺ����ᱵ�������������������Ϊ0.95��
�����ı�Һ�࣬���ⶨ�������ȴ��Ȼ�ƫ�ߣ��ʴ�Ϊ��ƫ�ߡ���֪��������������л��ܼ�A�У�����Ϊ5.39-5.26=0.03g����̼�����Ӧ��ֻ�б����Ӻ������ӣ�Ũ��Ϊ0.107mol/L��̼������Һ22.98mL�����ʵ���Ϊ0.00246mol�������ᱵ�������ȵ����ʵ���Ϊy��x����������ʽΪ212x+261y=5.26 x+y=0.0246�����������ȵ�������������5.39��ȥ����ƺ����ᱵ�������������������Ϊ0.95��
���㣺���⿼���������Ʊ�ʵ�鷽���ķ����жϣ�������������̣���ѧ��Ӧ���ʵ�Ӱ�����ط������ζ�ʵ��IJ����ָʾ��ѡ��������Ӧ�á�

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�ij��ѧʵ���Ҳ����ķ�Һ�к���Fe3����Cu2����Ba2����Cl���������ӣ���������з����Է�Һ���д������Ի��ս������Ʊ��Ȼ������Ȼ������塣
��1������1�к��еĽ��������� ��
��2������ʱ����H2O2��Һ������Ӧ�����ӷ���ʽΪ ��
��3�����������У�������Ϊ�Լ�X���� ������ĸ����
| A��BaCl2 | B��BaCO3 |
| C��NaOH | D��Ba(OH)2 |
��5���Ʊ��Ȼ�������������豣�������������Ŀ���� ��
��6���ɹ���2�õ�����Һ�Ʊ�BaCl2��ʵ���������Ϊ ����ȴ�ᾧ�� ��ϴ�ӡ����
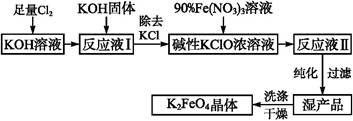
���������һ������Ч���ˮ����������ҵ�ϳ�����NaClO��������������Ӧԭ��Ϊ��
���ڼ��������£�����NaClO����Fe(NO3)3�Ƶ�Na2FeO4
3NaClO + 2Fe(NO3)3 + 10NaOH��2Na2FeO4��+ 3NaCl + 6NaNO3 + 5H2O
��Na2FeO4��KOH��Ӧ����K2FeO4��Na2FeO4 + 2KOH��K2FeO4 + 2NaOH
��Ҫ�������������£�
��1���������������ҺpHʱ����pH��ֽ���Բ���pH�Կ��Ƽ������������ʵ������pH��ֽ�ⶨ��ҺpH�IJ����� ��
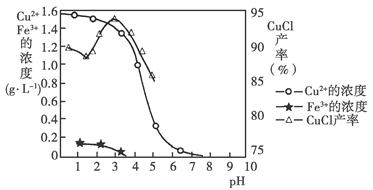
��2������ͼ�С�ת��������Ӧ�ۣ�����ij�����½��еģ�˵�����¶���Ksp��K2FeO4�� Ksp��Na2FeO4���������������������
��3����Ӧ���¶ȡ�ԭ�ϵ�Ũ�Ⱥ���ȶԸ�����صIJ��ʶ���Ӱ�졣
ͼ1Ϊ��ͬ���¶��£�Fe(NO3)3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죻
ͼ2Ϊһ���¶��£�Fe(NO3)3����Ũ�����ʱ��NaClOŨ�ȶ�K2FeO4�����ʵ�Ӱ�졣
��ҵ����������¶�Ϊ �棬��ʱFe(NO3)3��NaClO������Һ�������Ũ��֮��Ϊ ��
��4��K2FeO4��ˮ��Һ���ס�ˮ�⡱��4FeO42- + 10H2O  4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
| A��H2O | B��CH3COONa������� | C��NH4Cl������� | D��Fe(NO3)3������� |

 ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬���� ��Һ�����ƽ��ֵΪ19��00mL��
��Һ�����ƽ��ֵΪ19��00mL��
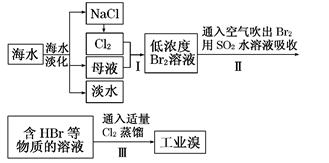
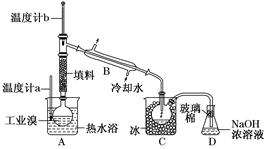

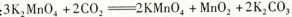
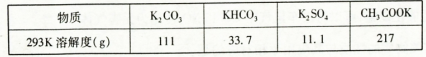
 KCl+KClO+H2O(����:�¶Ƚϵ�)
KCl+KClO+H2O(����:�¶Ƚϵ�) ;��������������������
;�������������������� 