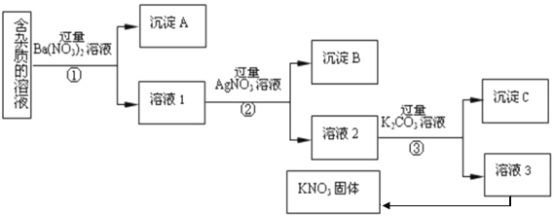
��Ŀ����
����Ŀ����������ĵ���ƽ�ⳣ�������
��1�������¢�0.1 mol��L��1 HCOONa����0.1 mol��L��1 NaClO����0.1 mol��L��1 Na2CO3����0.1 mol��L��1
NaHCO3��Һ��pH�ɴ�С�Ĺ�ϵΪ_____________________________________��
��2��Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2SO3��Na2CO3�Ļ����Һ�У�SO![]() ��CO
��CO![]() ��HSO
��HSO![]() ��HCO
��HCO![]() Ũ���ɴ�С��˳��Ϊ___________________________________��
Ũ���ɴ�С��˳��Ϊ___________________________________��
��3���������ӷ���ʽ��ȷ����________(����ĸ)��
a.2ClO����H2O��CO2===2HClO��CO![]() b.2HCOOH��CO
b.2HCOOH��CO![]() ===2HCOO����H2O��CO2��
===2HCOO����H2O��CO2��
c.H2SO3��2HCOO��===2HCOOH��SO![]() d.Cl2��H2O��2CO
d.Cl2��H2O��2CO![]() ===2HCO
===2HCO![]() ��Cl����ClO��
��Cl����ClO��
��4��ij�¶�(T ��)�µ���Һ�У�c(H��)��10��xmol��L��1��c(OH��)��10��y mol��L��1��x��y�Ĺ�ϵ��ͼ��ʾ.

�� ���¶��£�0.01mol/L��NaOH��Һ��ˮ�������OH-Ũ��Ϊ_____��
���ڴ��¶��£�0.1 mol��L��1��NaHSO4��Һ��0.1 mol��L��1��Ba(OH)2��Һ���±��мס��ҡ���������ͬ��ʽ��ϣ�
�� | �� | �� | �� | |
0.1 mol��L��1Ba(OH)2��Һ���/mL | 10 | 10 | 10 | 10 |
0.1 mol��L��1NaHSO4��Һ���/mL | 5 | 10 | 15 | 20 |
������ʽ��Ϻ�������Һ��________(����������������������)�ԣ�д�����ҷ�ʽ��Ϻ�Ӧ�����ӷ���ʽ��__________________________������ʽ��Ϻ�������Һ��pHΪ________��
���𰸡� 3241 SO32->CO32-> HCO3- >HSO3- bd 10-10mol/L ���� ���� 11
����������1����������Һ��Ϊǿ����������ˮ����Լ������γɸ��ε���Խ�������ε�ˮ������Խǿ�����Ծ�Խǿ���ӱ��еó����Դ�С��ϵΪ��HCOOH>H2CO3>HClO>HCO3-,��������Һ��pH�ɴ�С�Ĺ�ϵΪ��>��>��>�٣���ȷ�𰸣���>��>��>�١�
��2��������������Һ��Ϊǿ����������ˮ����Լ������γɸ��ε���Խ�������ε�ˮ������Խǿ�����Ծ�Խǿ���ӱ��еó����Դ�С��ϵΪ��HSO3->HCO3-,����Na2CO3��Һˮ������ǿ��ʣ���c��CO![]() ��Ũ��С�����ɵ�c(HCO
��Ũ��С�����ɵ�c(HCO![]() )���������������Ũ���ɴ�С��˳��Ϊ��SO32->CO32-> HCO3- >HSO3- ����ȷ����SO32->CO32-> HCO3- >HSO3-��
)���������������Ũ���ɴ�С��˳��Ϊ��SO32->CO32-> HCO3- >HSO3- ����ȷ����SO32->CO32-> HCO3- >HSO3-��
��3����������H2CO3>HClO>HCO3-,����ǿ���Ʊ����������ClO����H2O��CO2===HClO��HCO3-��a������������HCOOH> H2CO3������ǿ���Ʊ�������ɣ���Ӧ���Է�����b��ȷ����������.H2SO3> HCOOH> HSO3-��H2SO3�� HCOO��===HCOOH��HSO3-��c������������HCl>H2CO3>HClO>HCO3-,�÷�Ӧ�ܹ�������d��ȷ����ȷѡ��bd��
��4���ٸ���ͼ���֪��KW=10-12�����ݹ�ʽ����c(OH-)(��)+ c(OH-)(ˮ)����c(H��)��ˮ��= KW=10-12����0.01+ c(OH-)(ˮ)����c(H��)��ˮ��=10-12������c(OH-)(ˮ)= c(H��)��ˮ�������Խ��Ƽ���õ�c(OH-)(ˮ)= c(H��)��ˮ��=10-10 mol��L��1����ȷ��10-10 mol��L��1��
��0.1 mol��L��1��NaHSO4��Һ20 mL��0.1 mol��L��1��Ba(OH)2��Һ10 mL��Ϻ������Ӻ�����������ǡ����ȫ��Ӧ�����������ơ����ᱵ��ˮ����Һ�����������ҷ�ʽ��Ϻ�
������������ʣ�࣬��Һ�Լ��ԣ����ӷ���ʽΪBa2++SO42-+H++OH-=BaSO4��+H2O����ʽ��Ϻ���Һ�Լ��ԣ�ʣ��c(OH-)=��0.1��2��10��10-3-0.1��5��10-3��/(10+5)��10-3=0.1 mol��L��1,����KW=10-12������c(H��)=10-11 mol��L��1, ������Һ��pHΪ11����ȷ�𰸣����ԣ�11��

����Ŀ��ͼ�÷��෨��ʾ��һЩ���ʻ����֮��Ĵ����������ϵ������ȷ���ǣ�������
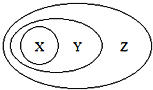
X | Y | Z | |
A | NaAlO2 | �� | ������ |
B | ���� | ��ɢϵ | ����� |
C | Al2O3 | ���������� | ������ |
D | ���ʲ��뷴Ӧ | �û���Ӧ | ������ԭ��Ӧ |
A. A B. B C. C D. D