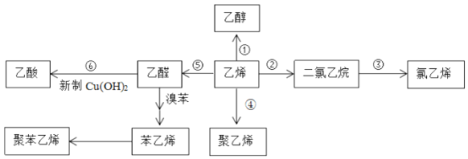
��Ŀ����
����Ŀ�����о����������ֵĹ����У�ͨ������������Cu��Ni��Zn��Sn��Fe����������
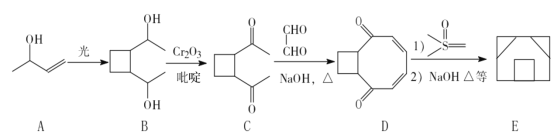
(1)ij�ֽ�������������Է��ԣ�ԭ������ά�ռ�����������������У��ý�������������________(���������������Ǿ�����)
(2)��̬Ni2���ĺ�������Ų�ʽΪ________��Ni2����Fe2���İ뾶�ֱ�Ϊ69 pm��78 pm�����۵�NiO______FeO(����<������>��)����ԭ����_________________________________________��
(3)ͭ������±��(SCN)2��Ӧ����Cu(SCN)2��1 mol (SCN)2�����к�����������ĿΪ________��д��һ����SCN����Ϊ�ȵ�����ķ���________(�û�ѧʽ��ʾ)��
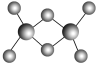
(4)��������ͭ�ķ��ӽṹ��ͼ��̼ԭ�ӵ��ӻ���ʽΪ________��

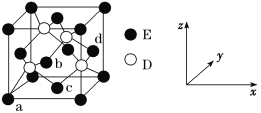
(5)����NiO(������)����Ľṹ��ͼ��ʾ���侧���߳�Ϊa pm����ʽ��ʾNiO������ܶ�Ϊ________g��cm��3(���ؼ��������������ӵ�������ֵΪNA)���˹��Ʊ���NiO�����г�����ȱ��(��ͼ)��һ��Ni2����ȱ����������Ni2��������Ni3����ȡ�������������Գʵ����ԣ�����������Ni��O�ı�ֵȴ�����˱仯����֪ij��������Ʒ���Ni0.96O���þ�����Ni3����Ni2�������Ӹ���֮��Ϊ________��

���𰸡����� 1s22s22p63s23p63d8��[Ar]3d8 > Ni2+��Fe2+���������������ͬ��Ni2+���Ӱ뾶��С��NiO�����Ӽ���ǿ��NiO������۵���� 5NA(��5��6.02��1023��3.01��1024) CO2 sp3��sp2 ![]() 1��11
1��11
��������
��1��ij�ֽ�������������Է��ԣ�ԭ������ά�ռ�����������������У��ý������������ھ��壻
�ʴ�Ϊ�����壻
��2��NiԪ��ԭ�Ӻ��������Ϊ28����������Ų�ʽΪ1s22s22p63s23p63d84s2��ʧȥ4s�ܼ�2�������γ�Ni2+����Ni2+���Ӻ�������Ų�Ϊ��1s22s22p63s23p63d8��
Ni2+��Fe2+���������������ͬ��Ni2+���Ӱ뾶��С��NiO�����Ӽ���ǿ��NiO������۵���ߣ����۵�NiO>FeO��
�ʴ�Ϊ��1s22s22p63s23p63d8��>��Ni2+��Fe2+���������������ͬ��Ni2+���Ӱ뾶��С��NiO�����Ӽ���ǿ��NiO������۵���ߣ�
��3��(SCN)2�ĽṹʽΪN��C-S-S-C��N������[(SCN)2]�Ľṹ��֪��������3��������2��̼������������ȫΪ��������������1��������2��������1����SCN��2���Ӻ���5����������1mol(SCN)2�����к�����������ĿΪ 5NA��
һ����SCN-��Ϊ�ȵ������ԭ��������ͬ���۵���������Ϊ16�����ϵķ�����CO2�ȣ�
�ʴ�Ϊ��5NA��CO2��
��4�����������Ӱ�����Cԭ���γ�2��C-H����1��C-N��1��C-C����û�йµ��Ӷԣ��ӻ������ĿΪ4����ȡsp3�ӻ�����̼��˫���е�Cԭ���γ�3��������û�йµ��Ӷԣ��ӻ������ĿΪ3����ȡsp2�ӻ���
�ʴ�Ϊ��sp3��sp2��
(5)������Ni2+��ĿΪ1+12��![]() =4��O2-��ĿΪ8��
=4��O2-��ĿΪ8��![]() +6��
+6��![]() =4����������Ϊ4��
=4����������Ϊ4��![]() g�������߳�Ϊapm���������Ϊ��a��10-10 cm��3��NiO������ܶ�ΪΪ4��
g�������߳�Ϊapm���������Ϊ��a��10-10 cm��3��NiO������ܶ�ΪΪ4��![]() g����a��10-10 cm��3=
g����a��10-10 cm��3=![]() g/cm3��
g/cm3��
��1mol Ni0.96O�к�Ni3+xmol��Ni2+Ϊ��0.96-x��mol�����ݾ����Գʵ����ԣ���֪ 3x+2����0.96-x��=2��1��x=0.08mol ��Ni2+Ϊ��0.96-x��mol=0.88mol����������֮��ΪNi3+��Ni2+=0.08��0.88=1��11��
�ʴ�Ϊ��![]() ��1��11��
��1��11��

����Ŀ��һ���¶��£��������ݻ���Ϊ2.0 L�ĺ����ܱ������з�����Ӧ��2NO(g)��2CO(g)![]() N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ����ͼ��ʾ������˵����ȷ����
N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ����ͼ��ʾ������˵����ȷ����
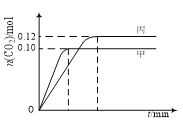
���� | �¶�/�� | ��ʼ���ʵ���/mol | |
NO (g) | CO (g) | ||
�� | T1 | 0.20 | 0.20 |
�� | T1 | 0.30 | 0.30 |
�� | T2 | 0.20 | 0.20 |
A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. �ﵽƽ��ʱ������CO2����������ȼ��еĴ�
C. T1��ʱ������ʼʱ����г���0.40 mol NO��0.40mol CO��0.40mol N2��0.40mol CO2����Ӧ�ﵽ��ƽ��ǰv(��)��v(��)
D. T2��ʱ������ʼʱ����г���0.06mol N2��0.12 mol CO2�����ƽ��ʱN2��ת���ʴ���40%