��Ŀ����
(1)������PH=2��ijǿ��ϡ��100����PH=_____��PH=12��ijǿ��ϡ��100����PH=____
(2)�����£�PH=2��HCl��PH=12�İ�ˮ�Ȼ���Ϻ���Һ��PH_ __7(��>,<��=),ԭ���� _______________________________________________________________________________
__7(��>,<��=),ԭ���� _______________________________________________________________________________
(3)�����£�0.01mol/L��HCl��0.01mol/L�İ�ˮ��Ϻ���Һ��PH___7(��>,<��=),ԭ����_______________________________________________________________________
(4)�����£���PH=5��H2SO4��Һϡ��10��C(H��)��C(SO42��)=_________
��ϡ�ͺ����Һ��ϡ��100����C(H��)��C(SO42��)=_________
(5)MOH��ROH����һԪ���ˮ��Һ�ֱ��ˮϡ��ʱ��PH�仯����ͼ��������������ȷ���ǣ���
A��MOH��һ������
B����x�㣬MOH��ȫ����
C����x��C(M��)=C(R��)
D:ϡ��ǰROH��Һ��C(OH��)��MOH��Һ��C(OH��)��10��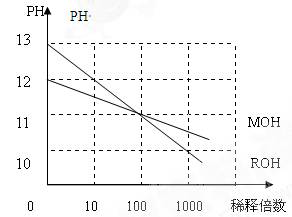
(2)�����£�PH=2��HCl��PH=12�İ�ˮ�Ȼ���Ϻ���Һ��PH_
 __7(��>,<��=),ԭ���� _______________________________________________________________________________
__7(��>,<��=),ԭ���� _______________________________________________________________________________(3)�����£�0.01mol/L��HCl��0.01mol/L�İ�ˮ��Ϻ���Һ��PH___7(��>,<��=),ԭ����_______________________________________________________________________
(4)�����£���PH=5��H2SO4��Һϡ��10��C(H��)��C(SO42��)=_________
��ϡ�ͺ����Һ��ϡ��100����C(H��)��C(SO42��)=_________
(5)MOH��ROH����һԪ���ˮ��Һ�ֱ��ˮϡ��ʱ��PH�仯����ͼ��������������ȷ���ǣ���
A��MOH��һ������
B����x�㣬MOH��ȫ����
C����x��C(M��)=C(R��)
D:ϡ��ǰROH��Һ��C(OH��)��MOH��Һ��C(OH��)��10��

(1) 4 ,  10 (
10 ( 2) > , ��Ӧ��ʣ
2) > , ��Ӧ��ʣ �������ˮ����ˮ����ʹ��Һ�ʼ���(3) < , ���ɵ�NH4Clˮ��ʹ��Һ������NH4����H2O
�������ˮ����ˮ����ʹ��Һ�ʼ���(3) < , ���ɵ�NH4Clˮ��ʹ��Һ������NH4����H2O NH3��H
NH3��H 2O��H�� (4)2��1 , 20��1 (5)B
2O��H�� (4)2��1 , 20��1 (5)B
 10 (
10 ( 2) > , ��Ӧ��ʣ
2) > , ��Ӧ��ʣ �������ˮ����ˮ����ʹ��Һ�ʼ���(3) < , ���ɵ�NH4Clˮ��ʹ��Һ������NH4����H2O
�������ˮ����ˮ����ʹ��Һ�ʼ���(3) < , ���ɵ�NH4Clˮ��ʹ��Һ������NH4����H2O NH3��H
NH3��H 2O��H�� (4)2��1 , 20��1 (5)B
2O��H�� (4)2��1 , 20��1 (5)B��

��ϰ��ϵ�д�
�����Ŀ
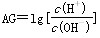 �� ���������������(����)
�� �����й������д������(����) ��AG��0��AGԽ����Һ��pHԽС
��AG��0��AGԽ����Һ��pHԽС
 ʱ���ų�445KJ�������������Ȼ�ѧ����ʽ��ȷ����
ʱ���ų�445KJ�������������Ȼ�ѧ����ʽ��ȷ����