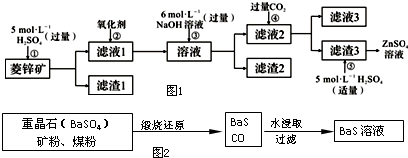
��Ŀ����
1��Ϊ�������ˮ��������������ͼ��ʾ��ʵ�飬�Թܼͱ�����60��80���ˮԡ����5��6min���Թ��Ҳ����ȣ����Թܼ��е���Һ��ȴ���ٽ��к���ʵ�飮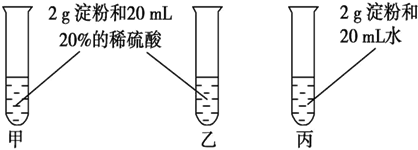
ʵ��1��ȡ����������Һ����������������ͭ�����ȣ�û��ש��ɫ�������֣�
ʵ��2��ȡ����������Һ���μӼ��ε�ˮ����Һ��Ϊ��ɫ����ȡ����������Һ����ʵ��ʱ����Һ������ɫ��
ʵ��3��ȡ����������Һ����NaOH��Һ���������ԣ��ٵμӵ�ˮ����Һ��ɫ�����Ա仯��
��1��д������ˮ��Ļ�ѧ����ʽ����C6H10O5��n�����ۣ�+nH2O$\stackrel{H+}{��}$nC6H12O6�������ǣ���
��2����Ƽ�����Ϊ��̽���¶ȶԵ���ˮ���Ӱ�죬��Ƽͱ���Ϊ��̽�������Ե���ˮ���Ӱ�죮
��3��ʵ��1ʧ�ܵ�ԭ����û�м�����к���Ϊ������ϡ���ᣮ
��4��ʵ��3����Һ����ɫ�����Ա仯��ԭ��������������ⷴӦ�ˣ�
���� ��1�����������Ի�����ˮ������ղ����������ǣ�
��2�������¶�Ϊʵ�������������̽���¶ȶԵ���ˮ���Ӱ�죬�ͱ������DZ���������������̽�������Ե���ˮ���Ӱ�죻
��3������ˮ���õ�����Һ�к���ϡ���ᣬ��Һ�����ԣ���Ӧ���ڼ��������½��У�
��4��������������ⵥ�ʷ�Ӧ�����ɵ⻯�ơ��ε����ƺ�ˮ��
��� �⣺��1���������ڶ��ǣ������Ի�����ˮ������ղ����������ǣ���ѧ����ʽΪ����C6H10O5��n�����ۣ�+nH2O$\stackrel{H+}{��}$nC6H12O6�������ǣ���
�ʴ�Ϊ����C6H10O5��n�����ۣ�+nH2O$\stackrel{H+}{��}$nC6H12O6�������ǣ���
��2���Թܼ��Ҿ���2g���ۺ�20mL20%��ϡ���ᣬ�Թܼ���60��80���ˮԡ����5��6min���Թ��Ҳ����ȣ�������Ƽ�����Ϊ��̽���¶ȶԵ���ˮ���Ӱ�죬�Թܱ���2g���ۣ���Ա�δ��20mL20%��ϡ���ᣬ�Թܼͱ�����60��80���ˮԡ����5��6min��������Ƽͱ���Ϊ��̽�������Ե���ˮ���Ӱ�죬
�ʴ�Ϊ���¶ȣ�������
��3������ˮ���õ�����Һ�к���ϡ���ᣬ��Һ�����ԣ���Ӧ���ڼ��������½��У���˵���������ϡ�������һ��ʱ��������һ���������������к�ϡ���ᣬ��Һ���ּ��ԣ�Ȼ���ټ�����������ͭ��Һ��
�ʴ�Ϊ��û�м�����к���Ϊ������ϡ���
��4��ʵ��3����Һ����ɫ�����Ա仯����Ϊ���ⵥ�ʺ�����������Һ��Ӧ���ɵ⻯�ơ��ε����ƺ�ˮ����ӦΪI2+2NaOH=NaI+NaIO+H2O��
�ʴ�Ϊ������������ⷴӦ�ˣ�
���� ���⿼�����ʵ�����ʵ�����ƣ�Ϊ��Ƶ���㣬�����л�������ʡ����鷽�������̷���Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��ҵ�����Ȼ�ԭ��ұ������Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$3CO2+2Fe | |
| B�� | ���Ṥҵ���ϳɰ���ҵ���ȼҵ��������ѭ���������ԭ�ϵ������� | |
| C�� | ���÷�Ӧ��NaCl�����ͣ�+CO2+NH3+H2O=NaHCO3��+NH4Cl��ȡNaHCO3ʱ��Ӧ��ͨ�������NH3����ͨ�������CO2 | |
| D�� | ���������г����ø�ѹ�������SO2��ת���� |
| A�� | ������Ư�۾�����������ˮ��ɱ�������� | |
| B�� | ���Ƶ�0.1mol/L��ˮ�м���������CaCO3���壬HClO�����ʵ���Ũ�ȼ�С | |
| C�� | Na2O��Na2O2������H2O��Ӧ���䷴Ӧԭ������ͬ | |
| D�� | O3��H2O2��SO2��Na2O2����Ư���ԣ���ԭ��������ͬ |
| A�� | ���³�ѹ�£�32g����������ԭ����ΪNA | |
| B�� | ��״���£�22.4L ���Ȼ�̼�ķ�����ΪNA | |
| C�� | 0�棬101KPa�£�44.8L���������еķ�����Ϊ2NA | |
| D�� | ���³�ѹ�£�1mol�������еķ�����Ϊ2NA |
| A�� | ��100-$\frac{7A}{6}$��% | B�� | 10A% | C�� | ��$\frac{A}{6}$��% | D�� | 6A% |
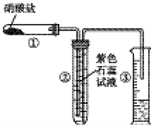 ijУ��ѧ��ȤС���ͬѧ�������ε����ȷֽ����̽���������������ͼװ�÷ֱ������NaNO3��Cu��NO3��2��AgNO3�������ȼ��г�װ��δ������
ijУ��ѧ��ȤС���ͬѧ�������ε����ȷֽ����̽���������������ͼװ�÷ֱ������NaNO3��Cu��NO3��2��AgNO3�������ȼ��г�װ��δ������ ���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮
���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮