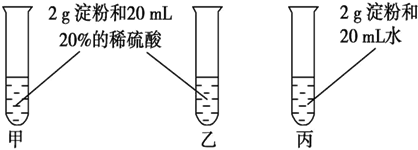
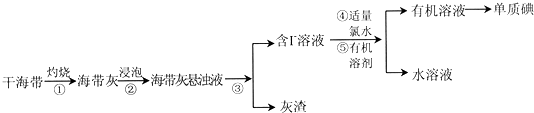
��Ŀ����
9�� ���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮
���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮��1��ͨ�������Ʋ⣺
��ԭ�����ҺNaCl��CuSO4�����ʵ���Ũ�ȣ�
��t2ʱ������Һ��pH��
��2��ʵ���з��֣��������������������������ȣ�����С�ڶ�Ӧʱ��ε�����ֵ���Լ�Ҫ���������ԭ��
���� ����Ĺؽ��Ƕ�ͼ��Ľ�����տ�ʼʱ����Cu2+�õ��ӣ�������ų���Cu2+��Ӧ����Һ�е�H+�ŵ磬����H2������������Һ�е�Cl-�ŵ磬��Ӧ�����Һ�е�OH-�ŵ磬���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ����������2Cl--2e-=Cl2����4OH--4e-=O2��+2H2O����������Cu2++2e-=Cu��2H++2e-=H2�������ͼ��֪����Ϊ�������������ʱ��Ĺ�ϵ����Ϊ�������������ʱ��Ĺ�ϵ������ʱץס�����غ㣮
��� �⣺��1�����200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ����������2Cl--2e-=Cl2����4OH--4e-=O2��+2H2O����������Cu2++2e-=Cu��2H++2e-=H2�������ͼ��֪����Ϊ�������������ʱ��Ĺ�ϵ����Ϊ�������������ʱ��Ĺ�ϵ��
����ͼ��֪����������Ϊ224mL������2Cl--2e-=Cl2����֪��n��NaCl��=$\frac{0.224L}{22.4L/mol}$��2=0.02mol������c��NaCl��=$\frac{0.02mol}{0.2L}$=0.1mol/L��
����������t2ʱ��������Ϊ112mL��n��O2��=$\frac{0.112L}{22.4L/mol}$=0.005mol����ת�Ƶ���Ϊ0.02mol+0.005mol��4=0.04mol��
���ݵ����غ㼰Cu2++2e-=Cu��֪��n��CuSO4��=$\frac{0.04mol}{2L}$=0.02mol������c��CuSO4��=$\frac{0.02mol}{0.2L}$=0.1mol/L��
��ԭ�����ҺNaCl��CuSO4�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��
����t2ʱ4OH--4e-=O2��+2H2O��4H+��n��H+��=0.005mol��4=0.02mol������Һ��c��H+��=$\frac{0.02mol}{0.2L}$=0.1mol/L��pH=1
����Һ��pH=1��
��2��������������������ˮ���ܽ�ȴ��������������ܽ�ȣ����������ܽ�ȴ������������������������������������ȣ�����С�ڶ�Ӧʱ��ε�����ֵ��
�𣺲�����������������ˮ�е��ܽ�����Դ���������
���� ���⿼����ԭ������ȷ�����ĵ缫��Ӧ��ͼͼ��Ķ�Ӧ��ϵ�ǽ����Ĺؼ���ע����յ缫��Ӧʽ����д��Ϊ�����Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | �ü�øϴ�·�ϴ�·�ʱ���¶�Խ��Ч��Խ�� | |
| B�� | �������ߺš��ķ������к����ķ���ϩ�����ķ���ϩ���ڲ������� | |
| C�� | ú����������Һ���������仯����ת��Ϊ���ȼ�� | |
| D�� | �Ҷ�������������������ά���������������Ŀ����� |
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04mol
��3�������ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ���ǣ�������
| A�� | Cl- �����ܴ��� | B�� | 100 mL��Һ�к�0.01 mol CO32- | ||
| C�� | K+һ������ | D�� | Ba2+һ�������ڣ�Mg2+���ܴ��� |
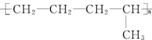 �ĵ�����CH2�TCH-CH3��CH2�TCH2����д�ṹ��ʽ��
�ĵ�����CH2�TCH-CH3��CH2�TCH2����д�ṹ��ʽ��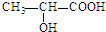 ���еĹ����������ǣ��ǻ����Ȼ���������������Ũ������ȵ������£�������һ�ֻ�״����д�������Ľṹ��ʽ��
���еĹ����������ǣ��ǻ����Ȼ���������������Ũ������ȵ������£�������һ�ֻ�״����д�������Ľṹ��ʽ��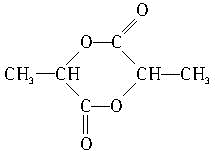 ��
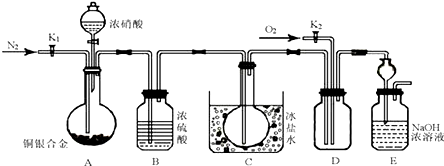
��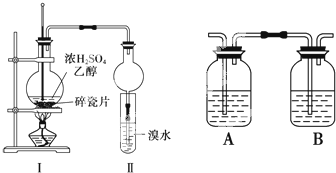 ij��ѧ��ȤС������ͼ��ʾװ�ý���̽��ʵ�飬����֤����������ϩ��������ϩ���в������ԣ�
ij��ѧ��ȤС������ͼ��ʾװ�ý���̽��ʵ�飬����֤����������ϩ��������ϩ���в������ԣ�