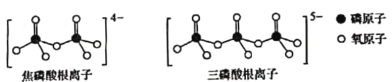
��Ŀ����
����Ŀ��ijͬѧ�������ͼ��ʾ��װ�ã��ɱȽ�HNO3��H2CO3��H2SiO3������ǿ���������Ƚϵ���̼����Ԫ�طǽ�����ǿ������ѡ����Լ���ϡ���ᡢϡ���ᡢ̼��ƹ��塢̼���ƹ��塢��������Һ������ʯ��ˮ������̼��������Һ
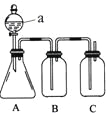
��1������a�����ƣ�________��
��2����ƿ��װ��ij���������Σ�a����ʢ�Լ�Ϊ________��
��3��װ��B��ʢ�Լ���____________________����������_____________��
��4��װ��C��ʢ�Լ���_______________��
C�з�Ӧ�����ӷ���ʽ��________________��
��5��ͨ��ʵ��֤��̼��������ķǽ�������ǿ������˳����________��
���𰸡���Һ©�� ϡ���� ����̼��������Һ �������������� ��������Һ SiO32����CO2��H2O��H2SiO3����CO32�� N��C��Si
��������
��1��a�����п��ع�Ϊ��Һ©����
��2����ƿ��װ��ij���������Σ�ֻ����̼���ƻ��߹����ƣ���Һ©����ֻ��ʢװ�ᣬ�����������У������������������֤����A��ʢװ̼������Һ��a��ʢװ���ᣬ������̼���Ʒ�Ӧ���������ơ�������̼��ˮ������ǿ���������ԭ������֪���ԣ�HNO3>H2CO3��
��3������������CO2��Ҫ��ȥ�����еĻӷ����������ᣬ���ñ���̼��������Һ���ӣ�
��4����һ����֤̼�����Դ��ڹ������ԣ��ʽ�������̼����ͨ���������Һ�У����ӷ���ʽΪ��SiO32����CO2��H2O��H2SiO3����CO32����
��5������������ˮ���������Խǿ����ӦԪ�صķǽ����Ծ�Խǿ���ʷǽ����ԣ�N��C��Si��