��Ŀ����
����Ŀ�������Τ��һ�ֺ�����������п��������ԣ�������״��������չ�ֳ��Ϻõ���Ч����ṹ��ͼ��ʾ��

�ش��������⣺
��1���ýṹ��̬Pԭ���У��������ռ������ܲ�ķ�����________________��ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ________________��
��2�������Τ��λ�ڵڶ�����Ԫ�صĵ�һ�����ܴӴ�С��˳��Ϊ________________�������е�ԭ�ӵ��ӻ�������________________��
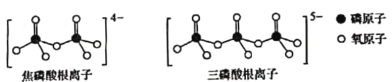
��3�����ӣ�![]() ���Ǻϳ������Τ��ԭ��֮һ�����۵�Ϊ43�������ӵľ���������________________��������ױ���
���Ǻϳ������Τ��ԭ��֮һ�����۵�Ϊ43�������ӵľ���������________________��������ױ���![]() ������Է�����������������ӵ��ۡ��е���ڼױ���ԭ����________________��
������Է�����������������ӵ��ۡ��е���ڼױ���ԭ����________________��
��4��MgSO4�Ǻϳ������Τ�Ĵ���֮һ��MgSO4�У������ӵĿռ乹��Ϊ________________��
��5������Ҳ�Ǻϳ������Τ��ԭ��֮һ��ֱ���Ķ����������ǣ��ָ��������Σ��磺�������ơ��������Ƶȡ�����������ӡ��������������ͼ��ʾ��
������������ӵĻ�ѧʽ����ͨʽ��ʾΪ________________����n����Pԭ��������
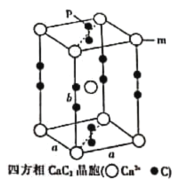
��6���ϳ������Τ��ԭ��֮һ�ı��ӿ�ͨ������;���Ƶã���ʯ��CaC2������ϩ�������屽�����ӡ��ķ���̼���ƣ�CaC2������ľ��ܽṹ��ͼ��ʾ���侧�������ֱ�Ϊapm��apm��bpm���ķ���̼���ƾ�����ܶ�Ϊ![]() g��cm-3��[C��C]2-�м���Ϊcpm�������ӵ�������ֵΪNA����mλ�õĸ�������Pλ�õ�̼ԭ��֮��ľ���Ϊ________________pm���ò���a�ļ������ʽ��ʾ����
g��cm-3��[C��C]2-�м���Ϊcpm�������ӵ�������ֵΪNA����mλ�õĸ�������Pλ�õ�̼ԭ��֮��ľ���Ϊ________________pm���ò���a�ļ������ʽ��ʾ����
���𰸡�M ���Ρ������� N>O>C sp3��sp2��sp ���Ӿ��� ���ӷ��Ӽ������� ���������� ��PnO3n+1)(n+2)- 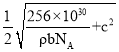
��������
(1)Pԭ��Ϊ��15��Ԫ�أ����������Ų�ʽΪ1s22s22p63s23p3����������ռ������ܲ�ķ�����M��ռ�ݸ��ܲ���ӷֱ�λ��3s��3p��������������״Ϊ���κ������Σ��ʴ�Ϊ��M�����Ρ������Σ�
(2)�ɽṹ������֪�������Τ�к��еĵڶ�����Ԫ����C��N��O������Nԭ�ӵļ۲����Ϊ2s22p3��2pΪ�����״̬������ʧȥ���ӣ����һ�����ܱ�����Ԫ�ش�Oԭ�Ӱ뾶С��Cԭ�ӣ���һ�����ܴ����һ�����ܣ�N>O>C���÷�����Nԭ�ӷֱ��γ��˵�����˫������������Nԭ�ӵ��ӻ���ʽ��sp3��sp2��sp���ʴ�Ϊ��N>O>C��sp3��sp2��sp��
(3)���ӵ��۵�Ϊ43������Խϵͣ���˱������ڷ��Ӿ��壻���ڱ��ӷ��Ӽ�����γ���������Ե��±��ӵ��ۡ��е���ڼױ����ʴ�Ϊ�����Ӿ��壻���ӷ��Ӽ���������
(4)MgSO4��������ΪSO42-��������ԭ��Sԭ�ӵļ۵��Ӷ���Ϊ![]() �������йµ��Ӷԣ�����ռ乹��Ϊ���������Σ��ʴ�Ϊ�����������Σ�
�������йµ��Ӷԣ�����ռ乹��Ϊ���������Σ��ʴ�Ϊ�����������Σ�
(5)����������������������������Ļ�ѧʽ(PO42-��P2O74-��P3O105-)���Ƶ�����ԭ�ӵı仯����Ϊ��1��2��3��4��n����ԭ�ӵı仯����Ϊ��4��7��10��3n+1������ı仯����Ϊ��3��4��5��n+2�����������������ӵĻ�ѧʽ����ͨʽ��ʾΪ��PnO3n+1)(n+2)-���ʴ�Ϊ����PnO3n+1)(n+2)-��
(6)���ݾ�̯����һ�������к���Ca2+����ĿΪ![]() ������C22-����ĿΪ
������C22-����ĿΪ![]() ����һ�������а�����2��CaC2������һ������������
����һ�������а�����2��CaC2������һ������������![]() ����
����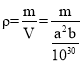 ����
���� �����mλ�õĸ�������Pλ�õ�̼ԭ��֮��ľ���Ϊ
�����mλ�õĸ�������Pλ�õ�̼ԭ��֮��ľ���Ϊ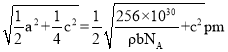 ���ʴ�Ϊ��
���ʴ�Ϊ��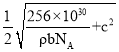 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ������ܴ��ʵ��Ŀ�����漰��������ԭ��Ӧ���ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ��ȥ | �Ⱥ�ͨ��ʢ������ |
B | ��ȥ | ������� |
C | ������Һ�к��� | ���� |
D | ����ϡ���������ˮ��IJ���Ϊ������ | ��ˮ������Һ��ֱ�Ӽ������� |
A.AB.BC.CD.D
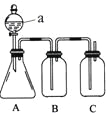
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50 mL0.25mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL0.55mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
��1����С�ձ��������ĭ���ϵ�������____��
��2������NaOH��Һ����ȷ������___��������ѡ������
A��һ��Ѹ�ٵ��� B���������������� C���ز�������������
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������____��
��4��ʵ���������±���
������д�±��еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2��t1��/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ___ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55mol/L NaOH��Һ��0.25mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/��g���棩�����к�����H��____�� ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��____��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d����ȡNaOH��Һ�����ʱ���Ӷ���