��Ŀ����
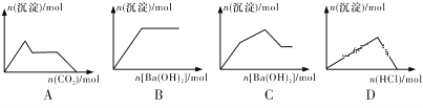
����Ŀ��2 L�ܱ������н��з�Ӧ��pZ(g)��qQ(g) ![]() mX(g)��nY(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ������ʵ����ʵ����ı仯���±���ʾ��
mX(g)��nY(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ������ʵ����ʵ����ı仯���±���ʾ��
���� | X | Y | Z | Q |
��ʼ/mol | 0.7 | 1 | ||
2 minĩ/mol | 0.8 | 2.7 | 0.8 | 2.7 |
3 minĩ/mol | 0.8 |
��֪��2 min��v(Q)��0.075 mol��L��1��min��1��v(Z)��v(Y)��1��2��
��ش��������⣺
(1)2 min��X�ķ�Ӧ����v(X)��__________��
(2)��ʼʱn(Y)��__________��
(3) 3 minĩ�Ƿ�ﵽƽ��_______(���ǻ��)��
(4)���ڸ÷�Ӧ������������Ӧ���ʵĴ�ʩ��________(����ţ���ͬ)��
A����С������� B�����߲���Q
C��ͨ�����He�� D�������¶�
���𰸡�0.025 mol��L��1��min��1 2.3 mol �� A D
��������
����ͼ�����ݼ���Ӧ����ʽ���㷴Ӧ���ʣ��������ݷ�����Ӧ�Ƿ�ﵽƽ�⡣
2 L�ܱ������н��з�Ӧ��pZ(g)��qQ(g) ![]() mX(g)��nY(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ������ʵ����ʵ����ı仯���±���ʾ��
mX(g)��nY(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ������ʵ����ʵ����ı仯���±���ʾ��
���� | X | Y | Z | Q |
��ʼ/mol | 0.7 | 1 | ||
2 minĩ/mol | 0.8 | 2.7 | 0.8 | 2.7 |
3 minĩ/mol | 0.8 |
��֪��2 min��v(Q)��0.075 mol��L��1��min��1��v(Z)��v(Y)��1��2��
��ش��������⣺
(1)2 min��X��Ũ��0.35 mol��L��1��Ϊ0.40 mol��L��1������v(X)��![]() =0.025 mol��L��1��min��1��
=0.025 mol��L��1��min��1��
�ʴ�Ϊ��0.025 mol��L��1��min��1��
(2)v(Z)��v(Y)��1��2����p��n=1��2��2min��Z��Ӧ��1mol-0.8mol=0.2mol����ͬʱ����YΪ0.2mol��2=0.4mol������ʼʱn(Y)��2.7mol-0.4mol=2.3 mol ��
�ʴ�Ϊ��2.3 mol ��
(3) 2minĩ��3 minĩZ�����ʵ�����ȣ���˵���Ѿ��ﵽƽ�⣬
�ʴ�Ϊ���ǣ�
(4)A����С�����������Ӧ��Ũ����������Ӧ���ʼӿ죬��A��ȷ��
B�����߲���Q����Ӧ��Ũ�ȼ�С������Ӧ���ʼ�С����B����
C��ͨ�����He������Ӧ��Ũ�Ȳ��䣬����Ӧ���ʲ��䣬��C����
D�������¶ȣ�����Ӧ���ʼӿ죬��D��ȷ��
�ʴ�Ϊ��AD��

 ��У����ϵ�д�
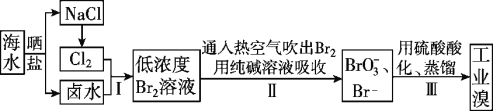
��У����ϵ�д�����Ŀ���������ʹ������������Դ���õ���Ҫ�о�����֮һ����ش��������⣺
(1)Ti(BH4)3��һ�ִ�����ϣ�����TiCl4��LiBH4��Ӧ�Ƶá�
�ٻ�̬Ti3+�ĵ����Ų�ʽΪ____________________��LiBH4��Li��B��HԪ�صĵ縺���ɴ�С������˳��Ϊ_________________��
������һ�ֺ���Ԫ�ص����Ͳ��ϣ������۽ṹģ����ͼ��ʾ��ͼ�����߿���̼ԭ�ӵ��ӻ��������Ϊ____________��

(2)������(NH3BH3)�������Ĵ�����ϣ��������������������(B2H6)��NH3�ϳɡ�
��NH3BH3���Ƿ������λ��__________(��ǡ���)����NH3BH3��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪ__________��
��B��C��N��OԪ�صĵ�һ�������ɴ�С��˳��Ϊ___________________��
�۰������ڸ������ͷ�������ɵ������������壬�������ƽ��ʯ�Ľṹ��Ӳ����С�ڽ��ʯ���������������������___________(ѡ��������ĸ���)��
a���������� b����̽��ͷ c��������� d����ĥ����
(3)һ���д���ܵ�ͭ�Ͻ�����������������ܶѻ��Ľṹ��������Cuԭ�Ӵ������ģ�Auԭ�Ӵ��ڶ���λ��,��ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�С�
������Cuԭ����Auԭ�ӵ�ͬ�������þ��崢���ľ����ṹ��CaF2�Ľṹ���ƣ��þ��崢���Ļ�ѧʽΪ________________��
��ͭ����������������仯���ﶼ���Է�����ɫ��Ӧ����ԭ����_______________��
(4)�����⻯��Ҳ�Ǿ������÷�չǰ���Ĵ�����ϡ�ij��������Ƕ����ڽ���Ԫ��R���⻯�R�IJ��ֵ��������±���ʾ��
I1/KJ��mol-1 | I2/KJ��mol-1 | I3/KJ��mol-1 | I4/KJ��mol-1 | I5/KJ��mol-1 |
738 | 1451 | 7733 | 10540 | 13630 |
�ٸý���Ԫ����___________(��Ԫ�ط���)����
�����⻯��ľ����ṹ��ͼ��ʾ(��4��Hԭ��λ�����ϣ�����Hԭ��λ�ھ�����)����֪�þ�����ܶ�Ϊ��g��cm-3,��þ��������Ϊ__________cm3[�ú��ѡ�NA�Ĵ���ʽ��ʾ(����NAΪ�����ӵ�������ֵ)]��

����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пհף�
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� |
��1������ЩԪ���У���ѧ������������ǣ�________(�����Ԫ�ط��ţ���ͬ)��
��2��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��_______��������ǿ�Ļ����������ʽ�ǣ�_______��
<>��3������������������������Ԫ����___��д���������������������Ʒ�Ӧ�����ӷ���ʽ______����4���õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣�__________���û���������_____(�� �����ۡ������ӡ�)�����
��5����ʾ����ߵĻ�����ĵ���ʽ______________���û���������_________������ԡ����Ǽ��ԡ���ͬ�����γɵġ�