ЬтФПФкШн
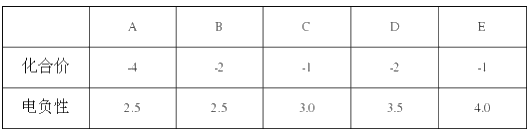
ЁОЬтФПЁПAЁЂBЁЂCЁЂD ОљЮЊЖЬжмЦкдЊЫизщГЩЕФЮяжЪЃЌЫќУЧжЎМфЗћКЯШчЯТзЊЛЏЙиЯЕЃК
![]()
ЃЈ1ЃЉШєA ЮЊПЩЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЕФЦјЬЌЧтЛЏЮяЃЌX ЮЊЫЎЃЌD ЮЊживЊЕФЛЏЙЄдСЯЁЃ
Ђй A ЕФЕчзгЪНЮЊ_________________ЃЌAЁњB ЕФЛЏбЇЗНГЬЪНЮЊ______________________ЁЃ
ЂкA КЭ D ЗЂЩњЛЏКЯЗДгІЕУЕНЕФЛЏКЯЮя E ЕФЛЏбЇЪНЪЧ___________ЃЌгУРызгЗНГЬЪНБэЪОМьбщEжабєРызгЕФЗНЗЈ________________ЁЃ
ЂлаДГіDЕФЯЁШмвКгыCuЗДгІЕФРызгЗНГЬЪН____________________________ЁЃ
ЃЈ2ЃЉШєAЮЊЗЧН№ЪєЕЅжЪЃЌXЮЊЫЎЃЌИУзЊЛЏЙиЯЕЮЊЙЄвЕЩњВњDЕФвЛЯЕСаЗДгІЁЃ
ЂйНЋBЭЈШыфхЫЎжабеЩЋЭЪШЅЃЌЬхЯжСЫBЕФ___________________адЃЌаДГіИУБфЛЏЕФЛЏбЇЗНГЬЪН________________________ЁЃ
ЂкAКЭH2ЗЂЩњЛЏКЯЗДгІЕУЕНЕФEЃЌдкBгыEЕФЗДгІжаЃЌбѕЛЏВњЮяКЭЛЙдВњЮяЕФжЪСПБШЮЊ_______________________________ЁЃ
ЂлаДГіDЕФХЈШмвКгыCuЗДгІЕФЛЏбЇЗНГЬЪН_________________________________________________________ЁЃ
ЃЈ3ЃЉШєAЮЊЬўЕФКЌбѕбмЩњЮяЃЌвНСЦЩЯГЃгУ75%ЃЈЬхЛ§ЗжЪ§ЃЉAЕФЫЎШмвКзїЯћЖОМСЃЌXЮЊМзДМЃЈCH3OHЃЉЁЃ
ЂйAЁњBЕФЛЏбЇЗНГЬЪНЮЊ____________________________ЃЛCЁњDЕФЛЏбЇЗНГЬЪНЮЊ_____________________________________ЁЃ
ЂкЯТСаЫЕЗЈе§ШЗЕФЪЧ___________________________________ЃЈбЁЬюађКХзжФИЃЉ
a. A ПЩЭЈЙ§ЦЯЬбЬЧЗжНтЗДгІЕУЕН b. B ЕФЙйФмЭХЕФНсЙЙМђЪНЮЊ-COH
c. C ПЩгы NaHCO3 ЗДгІВњЩњ CO2 d. A гыX ЛЅЮЊЭЌЯЕЮя
ЂлвбжЊЃКCHЁдCH дквЛЖЈЬѕМўЯТПЩгыCЗДгІЕУЕНEЃЈНсЙЙМђЪНЮЊ CH2=CHOOCCH3ЃЉЃЌдђЩЯЪіЗДгІЕФЗДгІРраЭЮЊ_______________________ЃЌEжаЕФЙйФмЭХУћГЦЮЊ_____________ЃЌEЗЂЩњМгОлЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________________________ЁЃ
ЁОД№АИЁП![]() 4NH3+5O2
4NH3+5O2![]() 4NO+6H2O NH3+HNO3=NH4NO3 NH4++OH-
4NO+6H2O NH3+HNO3=NH4NO3 NH4++OH-![]() NH3Ёќ+H2O 3Cu+8H++2NO3-=3Cu2+ +2NOЁќ+4H2O ЛЙд SO2+Br2+2H2O=2HBr+H2SO4 2:1 Cu+2H2SO4(ХЈ)
NH3Ёќ+H2O 3Cu+8H++2NO3-=3Cu2+ +2NOЁќ+4H2O ЛЙд SO2+Br2+2H2O=2HBr+H2SO4 2:1 Cu+2H2SO4(ХЈ)![]() CuSO4+SO2Ёќ+2H2O 2CH3CH2OH+O2
CuSO4+SO2Ёќ+2H2O 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3OH+CH3COOH
2CH3CHO+2H2O CH3OH+CH3COOH![]() CH3COOCH3+H2O acd МгГЩЗДгІ ѕЅЛљКЭЬМЬМЫЋМќ nCH2=CHOOCCH3
CH3COOCH3+H2O acd МгГЩЗДгІ ѕЅЛљКЭЬМЬМЫЋМќ nCH2=CHOOCCH3![]()
![]()
ЁОНтЮіЁП
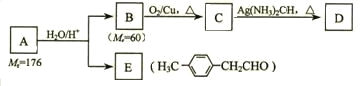
(1)ШєA ЮЊПЩЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЕФЦјЬЌЧтЛЏЮяЃЌдђAЮЊNH3ЃЌX ЮЊЫЎЃЌBЮЊNOЃЌCЮЊNO2ЃЌDЮЊHNO3ЃЌОнДЫЗжЮіНтД№ЃЛ
(2)ШєAЮЊЗЧН№ЪєЕЅжЪЃЌXЮЊЫЎЃЌНЋBЭЈШыфхЫЎжабеЩЋЭЪШЅЃЌдђAЮЊSЃЌBЮЊSO2ЃЌCЮЊSO3ЃЌDЮЊH2SO4ЃЌОнДЫЗжЮіНтД№ЃЛ
(3)ШєAЮЊЬўЕФКЌбѕбмЩњЮяЃЌвНСЦЩЯГЃгУ75%(ЬхЛ§ЗжЪ§)AЕФЫЎШмвКзїЯћЖОМСЃЌдђAЮЊввДМ(CH3CH2OH)ЃЌXЮЊМзДМ(CH3OH)ЃЌдђBЮЊввШЉЃЌCЮЊввЫсЃЌDЮЊввЫсМзѕЅЃЌОнДЫЗжЮіНтД№ЁЃ
(1)ШєA ЮЊПЩЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЕФЦјЬЌЧтЛЏЮяЃЌдђAЮЊNH3ЃЌX ЮЊЫЎЃЌBЮЊNOЃЌCЮЊNO2ЃЌDЮЊHNO3ЃЌ
ЂйA ЕФЕчзгЪНЮЊ![]() ЃЌAЁњB АБЦјЕФДпЛЏбѕЛЏЃЌЛЏбЇЗНГЬЪНЮЊ4NH3+5O2
ЃЌAЁњB АБЦјЕФДпЛЏбѕЛЏЃЌЛЏбЇЗНГЬЪНЮЊ4NH3+5O2![]() 4NO+6H2OЃЛ
4NO+6H2OЃЛ
ЂкNH3КЭHNO3ЗЂЩњЛЏКЯЗДгІЕУЕНЕФЛЏКЯЮяЯѕЫсяЇЃЌЛЏбЇЪНЪЧNH3+HNO3=NH4NO3ЃЌМьбщяЇИљРызгЕФЗНЗЈШЁЩйСПД§ВтвКЬхгкЪдЙмжаЃЌЕЮМгЧтбѕЛЏФЦШмвКВЂМгШШЃЌгУЪЊШѓЕФКьЩЋЪЏШяЪджНгкЪдЙмПкЃЌЪджНБфРЖЃЌжЄУїгаАБЦјЩњГЩЃЌНјЖјжЄУїгаяЇИљРызгДцдкЃЌЗЂЩњЕФРызгЗДгІЮЊЃКNH4++OH-![]() NH3Ёќ+H2OЃЛ
NH3Ёќ+H2OЃЛ
ЂлЯЁЯѕЫсгыCuЗДгІЩњГЩЯѕЫсЭЁЂвЛбѕЛЏЕЊКЭЫЎЃЌРызгЗНГЬЮЊ3Cu+8H++2NO3-=3Cu2+ +2NOЁќ+4H2OЃЛ
(2) ШєAЮЊЗЧН№ЪєЕЅжЪЃЌXЮЊЫЎЃЌНЋBЭЈШыфхЫЎжабеЩЋЭЪШЅЃЌдђAЮЊSЃЌBЮЊSO2ЃЌCЮЊSO3ЃЌDЮЊH2SO4ЃЛ
ЂйНЋSO2ЭЈШыфхЫЎжаЗЂЩњбѕЛЏЛЙдЗДгІЩњГЩСђЫсКЭфхЛЏЧтЃЌфхЫЎбеЩЋЭЪШЅЃЌЬхЯжСЫSO2ЕФЛЙдадЃЌИУБфЛЏЕФЛЏбЇЗНГЬЪНSO2+Br2
ЂкSКЭH2ЗЂЩњЛЏКЯЗДгІЕУЕНЕФH2SЃЌдкH2SгыSO2ЕФЗДгІжаЩњГЩSКЭЫЎЃЌЗДгІЗНГЬЪНЮЊЃК2H2S+SO2=3S+2H2OЃЌH2SжаЕФSЛЏКЯМлЩ§ИпЃЌБЛбѕЛЏЃЌSO2жаЕФSдЊЫиЛЏКЯМлНЕЕЭЃЌБЛЛЙдЃЌбѕЛЏВњЮяКЭЛЙдВњЮяЕФжЪСПБШЮЊ2:1ЃЛ
ЂлХЈСђЫсгыCuдкМгШШЬѕМўЯТЗДгІЩњГЩСђЫсЭЁЂЖўбѕЛЏСђКЭЫЎЃЌЛЏбЇЗНГЬЪНCu+2H2SO4(ХЈ)![]() CuSO4+SO2Ёќ+2H2OЃЛ
CuSO4+SO2Ёќ+2H2OЃЛ
(3) ШєAЮЊЬўЕФКЌбѕбмЩњЮяЃЌвНСЦЩЯГЃгУ75%(ЬхЛ§ЗжЪ§)AЕФЫЎШмвКзїЯћЖОМСЃЌЃЌдђAЮЊввДМ(CH3CH2OH)ЃЌXЮЊМзДМ(CH3OH)ЃЌдђBЮЊввШЉЃЌCЮЊввЫсЃЌDЮЊввЫсМзѕЅЃЛ
ЂйввДМдкДпЛЏМСМгШШЬѕМўЯТгыбѕЦјЗЂЩњДпЛЏбѕЛЏЗДгІЃЌЛЏбЇЗНГЬЪНЮЊ2CH3CH2OH+O2![]() 2CH3CHO+2H2OЃЛCЮЊввЫсЃЌXЮЊМзДМ(CH3OH)ЃЌввЫсКЭМзДМдкХЈСђЫсМгШШЬѕМўЯТЗЂЩњѕЅЛЏЗДгІЃЌЛЏбЇЗНГЬЪНЮЊCH3OH+CH3COOH
2CH3CHO+2H2OЃЛCЮЊввЫсЃЌXЮЊМзДМ(CH3OH)ЃЌввЫсКЭМзДМдкХЈСђЫсМгШШЬѕМўЯТЗЂЩњѕЅЛЏЗДгІЃЌЛЏбЇЗНГЬЪНЮЊCH3OH+CH3COOH![]() CH3COOCH3+H2OЃЛ
CH3COOCH3+H2OЃЛ
ЂкaЃЎA ЮЊввДМЃЌЮобѕЛђШБбѕЕФЬѕМўЯТЃЌЭЈЙ§УИЕФДпЛЏзїгУЃЌАбЦЯЬбЬЧЕШгаЛњЮяВЛГЙАйЕзЕФбѕЛЏЗжНтГЩОЦОЋЛђШщЫсЕШЃЌЗДгІЮЊC6H12O6![]() 2C2H5OH+ 2CO2ЃЌЙЪaе§ШЗЃЛ
2C2H5OH+ 2CO2ЃЌЙЪaе§ШЗЃЛ
bЃЎBЮЊввШЉЃЌдђBЕФЙйФмЭХЕФНсЙЙМђЪНЮЊ-CHOЃЌЙЪbДэЮѓЃЛ
cЃЎCЮЊввЫсЃЌввЫсЕФЫсадЧПгкЬМЫсЃЌдђCПЩгыNaHCO3ЗДгІВњЩњCO2ЃЌЙЪcе§ШЗЃЛ
dЃЎНсЙЙЯрЫЦЃЌРрБ№ЯрЭЌЃЌЗжзгзщГЩЩЯЯрВювЛИіЛђЖрИі-CH2-ЕФгаЛњЮяЛЅЮЊЭЌЯЕЮяЃЌAЮЊввДМ(CH3CH2OH)ЃЌXЮЊМзДМ(CH3OH)ЃЌЛЅЮЊЭЌЯЕЮяЃЌЙЪdе§ШЗЃЛ
Д№АИбЁacdЃЛ
ЂлвбжЊЃКCHЁдCH дквЛЖЈЬѕМўЯТПЩгыCЗДгІЕУЕНE(НсЙЙМђЪНЮЊCH2=CHOOCCH3)ЃЌШ§МќБфЫЋМќЃЌЗДгІРраЭЮЊМгГЩЗДгІЃЌEжаЕФЙйФмЭХУћГЦЮЊѕЅЛљКЭЬМЬМЫЋМќЃЌEЗЂЩњМгОлЗДгІЕФЛЏбЇЗНГЬЪНЮЊnCH2=CHOOCCH3![]()
![]() ЁЃ
ЁЃ