��Ŀ����
����Ŀ��������֪��R��CH��CH��O��R��![]() R��CH2CHO + R��OH
R��CH2CHO + R��OH
������ϩ���ѣ�
����ϩ����A����Է���������Mr��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ����A��صķ�Ӧ���£�
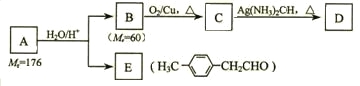
��ش��������⣺
�� A�ķ���ʽΪ_________________��
�� B��������__________��A�Ľṹ��ʽΪ_______________��
��д��C �� D��Ӧ�Ļ�ѧ����ʽ��_____________________________��
��д������ͬʱ��������������E��ͬ���칹��Ľṹ��ʽ��
�����ڷ���ȩ�� �ڱ����������ֲ�ͬ��������ԭ�ӡ�
_________________________��________________________��
������Eת��Ϊ�Լ�����Ȳ��![]() ����һ��·�����£�
����һ��·�����£�

��д��G�Ľṹ��ʽ��___________________________��
��д����-�ܲ���Ӧ�����Լ�����Ӧ��������-������Ӧ���ͣ�____________
��� | �����Լ�����Ӧ���� | ��Ӧ���� |
�� | ||
�� | ||
�� | ||
�� | ���� |
���𰸡�C12H16O 1-���������������� ![]() CH3CH2CHO+2Ag��NH3��2OH
CH3CH2CHO+2Ag��NH3��2OH![]() CH3CH2COONH4+2Ag��+3NH3+H2O
CH3CH2COONH4+2Ag��+3NH3+H2O 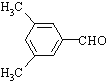
 ��
��![]()
![]()
��� | �����Լ�����Ӧ���� | ��Ӧ���� |
�� | H2����������Ni��Pt��Pd������ | ��ԭ����ӳɣ���Ӧ |
�� | ŨH2SO4���� | ��ȥ��Ӧ |
�� | Br2����Cl2�� | �ӳɷ�Ӧ |
�� | NaOH��C2H5OH���� | ���� |
��������
��������Ϣ��֪A�ķ���ʽ��дΪ��C3H4��nO����40n+16=176��n=4������A�ķ���ʽΪC12H16O����ת��ͼ��֪B��̼ԭ����Ϊ3���ô��������ɵ�C���ܹ�����������Ӧ����B���ǻ��ڶ˵㣬��B��1-������CΪCH3CH2CHO��DΪCH3CH2COONH4�����ƿ�֪A�Ľṹ��ʽΪ![]() ��
��
�����ɺ���̼̼���������ʣ�һ��Ӧ��ȡ±��������ȥ��Ӧ���ʵڢٲ���ȩ�ӳ�Ϊ�����ڢڲ��Ǵ���ȥ��ϩ�����ڢ۲�����Br2�ӳɣ��ڢܲ���±��������ȥ��������ʵĽṹ���ʽ����⡣
��1��A�ķ���ʽ��дΪ��C3H4��nO����40n+16=176��n=4������A�ķ���ʽΪC12H16O���ʴ�Ϊ��C12H16O��
��2����B/span>�ɴ�������ȩ����Է�������Ϊ60��֪��BΪ����������B��E��������Ϣ�����ƿ�֪A�Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��1-������������������![]() ��
��
��3��C��D��������������Ӧ����Ӧ����ʽΪ��CH3CH2CHO+2Ag��NH3��2OH![]() CH3CH2COONH4+2Ag��+3NH3+H2O��
CH3CH2COONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3CH2CHO+2Ag��NH3��2OH![]() CH3CH2COONH4+2Ag��+3NH3+H2O��
CH3CH2COONH4+2Ag��+3NH3+H2O��
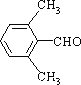
��4��EΪ![]() �����ϱ����������ֲ�ͬ����Hԭ�ӵĽṹ�ԳƳ̶�Ӧ�ϸߣ��У�
�����ϱ����������ֲ�ͬ����Hԭ�ӵĽṹ�ԳƳ̶�Ӧ�ϸߣ��У�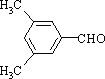 ��
�� ��
��![]() ��
��
�ʴ�Ϊ��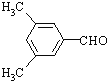 ��
��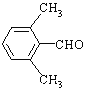 ��
��![]() ��
��
��5��������ղ���![]() ��֪��GӦ����ͬ��̼�ܽṹ��������Է�������Ϊ118��֪EӦΪ
��֪��GӦ����ͬ��̼�ܽṹ��������Է�������Ϊ118��֪EӦΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��6�����ɺ���̼̼���������ʣ�һ��Ӧ��ȡ±��������ȥ��Ӧ���ʵڢٲ���ȩ�������ڴ����������¼ӳ�Ϊ�����ڢڲ��Ǵ���Ũ���������¼�����ȥ��ϩ�����ڢ۲�����Br2�ӳɣ��ڢܲ���±������NaOH���Ҵ���Һ�з�����ȥ����������£�
��� | �����Լ�����Ӧ���� | ��Ӧ���� |
�� | H2����������Ni��Pt��Pd������ | ��ԭ����ӳɣ���Ӧ |
�� | ŨH2SO4���� | ��ȥ��Ӧ |
�� | Br2����Cl2�� | �ӳɷ�Ӧ |
�� | NaOH��C2H5OH���� | ���� |
�ʴ�Ϊ��
��� | �����Լ�����Ӧ���� | ��Ӧ���� |
�� | H2����������Ni��Pt��Pd������ | ��ԭ����ӳɣ���Ӧ |
�� | ŨH2SO4���� | ��ȥ��Ӧ |
�� | Br2����Cl2�� | �ӳɷ�Ӧ |
�� | NaOH��C2H5OH���� | ���� |




