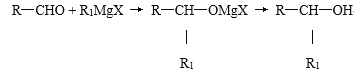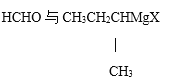��Ŀ����
����Ŀ����ͼ��������ϸ�������ﺬ��������ͼ��ͼ��Ϊ���������Ⱦɫ���������Ҫ������A��C�Ļ�ѧ��ɹ�ϵ����ͼ�����ش�
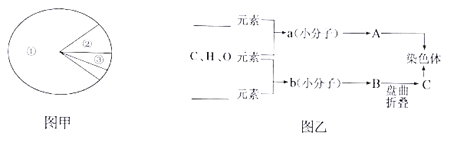
��1����ͼ�ױ�ʾϸ�����أ���ٻ�������_______����ͼ�ױ�ʾϸ�����أ���ٻ�������________������ͼ���е�������_______������ĸ��ʾ����
��2��ͼ����b����ͨ��______��ʽ�γ�B����������b���ӵĻ�ѧ������_______�����ýṹʽ��ʾӦΪ__________��
��3����ͼ���У�a��b���ֻ��������������Ԫ�طֱ���_______��_______�������ﵥ��a��������________________��
��4������֪���˵ĺ�ϸ�����ļ�ϸ������Ҫ�ɷֶ��ǵ����ʣ���������ϸ���Ĺ���ȴ��ȫ��ͬ������ӵ����ʵĽṹ������������_______________________________��
���𰸡�ˮ ������ C ��ˮ���� �ļ� -CO-NH- N��P N �������Ǻ����� ������ϸ���й��ɵ����ʵİ���������ࡢ��Ŀ������˳���Լ������ʵĿռ�ṹ��ͬ
��������
����ʱ���ȸ����л�����ӵĹ���ȷ������ӵ����࣬����ȷ��������λ������ϸ���к������Ļ�������ˮ������ǵ����ʣ���֪ͼ���Т���ˮ�����ǵ����ʡ�
����ͼ��A��C����Ⱦɫ�壬��֪AΪDNA�� BΪ���ģ�C�ǵ����ʣ��������λbΪ�����A�Ļ�����λaΪ���������ᡣ
��1��ϸ������ʱ����������ˮ������ǵ����ʣ��ʢ���ˮ�����ǵ����ʡ�ϸ������ʱ�������Ļ�����Ϊ�����ʣ�������ͼ�ױ�ʾϸ�����أ���ٻ������ǵ����ʣ�����ͼ���е�������C������B�������۵��γɣ���
��2��ͼ����bΪ�����ᣬͨ����ˮ���ϵķ�ʽ�γ�B���ģ���������������Ļ�ѧ�������ļ����ṹʽ�ɱ�ʾΪ-CO-NH-��
��3��������������֪��a���������Ǻ����ᣬ���Ԫ��ΪC��H��O��N��P��b�ǰ����ᣬ���Ԫ��ΪC��H��O��N������a��b���ֻ��������������Ԫ�طֱ���N��P��N��
��4����ͬϸ�����ܲ�ͬ��ֱ��ԭ����ϸ���е����ʵĹ��ܲ�ͬ���������ʹ��ܲ�ͬ��ԭ��������ɵ����ʵİ���������ࡢ��Ŀ������˳���Լ������ʵĿռ�ṹ��ͬ��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�����Ŀ�����շ��Σ�Mn(H2PO4)2����һ�ְ�ɫ���壬������ˮ�������ڻ�е�豸���������������̿���Ҫ�ɷ�ΪMnO2��������������Fe2O3��FeO��Al2O3��Ϊԭ���Ʊ����շ��ε��������£�

��1���������̿��Ƴɿ�Ŀ����_________________________________________�������ǣ�C6H12O6����MnO2��Ӧʱ������ΪMnSO4��CO2��H2O���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ__________��
��2����H2O2��Һ��������ʱ������Ӧ�����ӷ���ʽΪ_______________________________��
��3����֪���ֽ������ӵ��������↑ʼ��������ȫ������pH���±���ʾ������pH��������ʱ��Ӧ������pH��ΧΪ____________������1����Ҫ�ɷ�Ϊ_________���ѧʽ����
�������� | ��ʼ������pH | ��ȫ������pH |
Fe3+ | 1.8 | 3.2 |
Al3+ | 3.0 | 5.0 |
Fe2+ | 5.8 | 8.8 |
Mn2+ | 7.8 | 9.8 |
��4���������������Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��5��ij���������������Ʊ����շ��Σ���֪���̿���MnO2�ĺ���Ϊ87%��������������Ԫ�ص������Ϊ9%����1t�����̿���Ƶ����շ���__________t��
����Ŀ����ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����
![]()
ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
1 | 10 mLFeSO4��Һ | 10 mLNH3 | ���ɰ�ɫ���������ɫ |
2 | 20 mLH2S | 12mLSO2 | |
3 | 30 mLNO2 | 10 mLH2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
4 | 15 mLCl2 | 40 mLNH3 |
�Իش��������⣺
(1)ʵ��1�У��������ձ�Ϊ_____ɫ��д��������ɫ�Ļ�ѧ����ʽ_________________________��
(2)ʵ��2����Ͳ�ڵ������ǣ���_____________���ɣ�����______�ƶ��������⡢���ڡ�������
��Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
(3)ʵ��3�У��������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽ_______��
(4)ʵ��4�У���֪��Cl2 + NH3 �� N2+ HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ____________�������Ͳ��ʣ����������ԼΪ_________mL��
���𰸡����ɫ4Fe(OH)2��O2��2H2O=4Fe(OH)3����ɫ��������NaOHNO3NO2+H2O = 2HNO3+NO����ɫ��Ϊ��ɫ5
��������
+2�۵��������ױ�����������+3�۵������ӣ�NH3��FeSO4��Һ��ˮ��Ӧ����Fe��OH��2������Fe��OH��2�����ױ������е�����������H2S��SO2��Ӧ���ɵ������ˮ��������SO2�ü����գ�NO2��ˮ��Ӧ���������һ����������������ɫΪ����ɫ����������������������ԭ��Ӧ���ɵ������Ȼ��⣬3Cl2+2NH3�TN2+6HCl�������İ���������Ȼ��ⷴӦ�����Ȼ�李�
��1����������ˮ�õ���ˮ����Ӧ�Ļ�ѧ����ʽΪ��NH3+H2ONH3H2O����ˮ������������Һ��Ӧ����������������ɫ��������Ӧ�Ļ�ѧ����ʽΪ��FeSO4+2NH3H2O�TFe��OH��2��+��NH4��2SO4��Fe��OH��2��ɫ�������ڿ������ױ������е�����������Ѹ�ٱ�ɻ���ɫ�����ձ�Ϊ���ɫ����Ӧ�Ļ�ѧ����ʽΪ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3����2��15mLH2S��10mLSO2��Ӧ2H2S+SO2=3S+2H2O����Ӧ�����������Һ̬ˮ��SO2��������Ͳ��ѹǿ��С�������Զ������ƶ�������dz��ɫ���壬������SO2��NaOH��Һ���ա���3������������������ˮ����ˮ��Ӧ���������һ����������Ӧ�Ļ�ѧ����ʽΪ��3NO2+H2O=2HNO3+NO���ɷ���ʽ��֪��֪30 mLNO2����10mL��ɫ��NO���塣��4����������ɫΪ����ɫ����������������������ԭ��Ӧ���ɵ������Ȼ��⣬������ɫ��dz����Ӧ�Ļ�ѧ����ʽΪ��3Cl2span>+2NH3�TN2+6HCl���ɷ���ʽ��֪��15 mLCl2����10ml����������30ml�Ȼ��⣬�����İ������Ȼ���ǡ�÷�Ӧ�����Ȼ�泥���Ӧ���д����İ��̲�����
���㾦��
������Ҫ������Ԫ�ؼ��仯�����֪ʶ���漰�����ȡ���Ļ���������ʣ�ע����ݻ������������ʶ���⻯ѧ��Ӧ����ȷ����ʵ������
�����͡������
��������
30
����Ŀ����һƿ���������Ļ����Һ(1)ȡ��10.00mL����Һ����������BaCl2��Һ�����ˡ�ϴ�ӡ������4.66g��������Һ��40 mL 2.00mol/L��NaOH��Һǡ�÷�Ӧʹ��Һ�����ԣ�����Һ���������������ʵ���Ũ�ȷֱ�Ϊ����____��(2)ȡ��20.00mL����Һ����������Cu�ۣ�����������ֻ��NO���ɲ���NO�����ʵ����Ƕ���____ ��
����Ŀ����֪��I2+2S2O32����S4O62��+2I����������ʵ��ܶȻ��������±���
���� | Cu(OH)2 | Fe(OH)3 | CuCl | CuI |
Ksp | 2.2��10��20 | 2.6��l0��39 | 1.7��l0��7 | 1.3��l0��12 |
��1��ij����CuCl2��Һ�к���������FeCl3��Ϊ�õ�������CuC12��2H2O���壬������CuO��Cu(OH)2������pH��4��ʹ��Һ�е�Fe3+ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3+)��_____________�� ���˺�������Һ����������Ũ���ᾧ���ɵõ�CuCl2��2H2O���塣
��2���ڿ�����ֱ�Ӽ���CuCl2��2H2O����ò���������ˮCuC12�� ԭ����(�û�ѧ����ʽ��ʾ)_____________________________����CuCl2��2H2O����õ���������ˮCuCl2�ĺ���������________________________________________________��
��3��ijѧϰС���á���ӵ��������ⶨ����CuCl2��2H2O���������(��������I��������Ӧ������������)�Ĵ��ȣ��������£�ȡ1.44g��������ˮ���������KI���壬��ַ�Ӧ�����ɰ�ɫ����(CuI)������Ӧ�����Һϡ��Ϊ100 mL����0.1000mol/LNa2S2O3����Һ����Һ�������ԣ����еζ����ζ�������±���ʾ��
�ζ����� | ������Һ�����/mL | ����Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.02 | 22.03 |
2 | 25.00 | 2.00 | 21.98 |
3 | 25.00 | 0.20 | 20.22 |
�ٿ�ѡ��__________���ζ�ָʾ�����ζ��յ��������_________________________��
�ڵζ������в����ζ��ܵ�ͼʾ��ȷ����_____________��
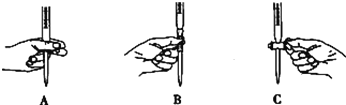
�����ζ�ʱ��������������ⶨ���ƫ�ߵ���___________��
a���ζ�ǰ������ˮ��ϴ��ƿ b���ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ
c����ʽ�ζ��ܵ����յ�ʱ���Ӷ��� d����ʽ�ζ�������ˮϴ��δ�ñ�Һ��ϴ
��CuCl2��Һ��KI��Ӧ�����ӷ���ʽΪ______________________________��
�ݸ�������CuCl2��2H2O�������ٷ���Ϊ____________________��